Bexion Pharmaceuticals received Orphan Drug Designation for its novel drug, BXQ-350 for the treatment of patients with glioblastoma multiforme. The drug has shown activity in over 60 different cancer cell lines, and is toxic to cancer cells, only.The nanovesicals bypass the blood brain barrier.
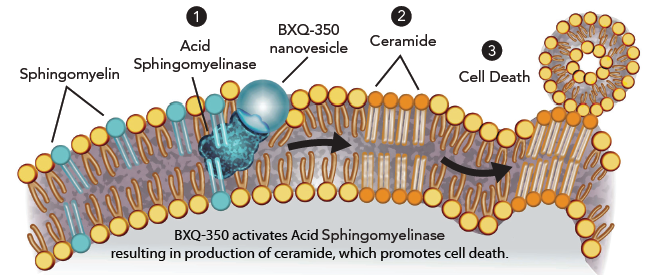
BXQ-350 has an entirely new and novel mechanism of action. Studies strongly suggest that specific tumor targeting is the result of targeting phosphatidylserine patches that are characteristic of tumor cells and neovasculature; and that apoptosis results from activation of acid sphingomyelinase, subsequent generation of ceramide, and elevated levels of caspase.
Orthotopic glioma models in mice demonstrate activity of BXQ-350.
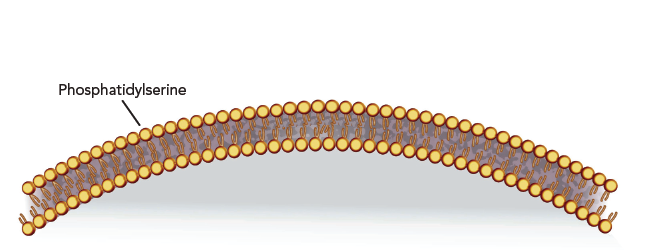
When healthy cells turn cancerous they undergo a series of physiological changes. During this transition, a fat molecule called phosphatidylserine (PS) flips from just inside the cell’s membrane to its outside surface.
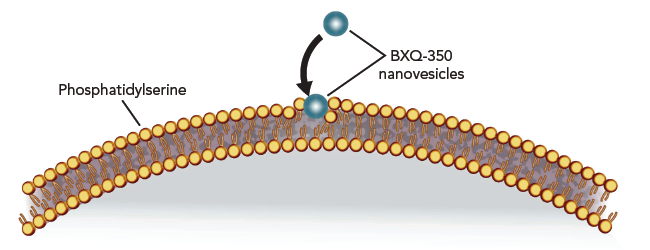
All cancer cells appear to “externalize” PS. This attribute may help tumor cells evade the body’s immune defense system and could promote metastases. Bexion’s lead compound, BXQ-350, targets this externalized PS.
Bexion’s lead compound, BXQ-350, targets this externalized PS, and subsequently catalyzes a series of biochemical reactions in the tumor cell that causes ceramide to be released, which, in turn results in apoptosis.
Ceramide exerts its cytotoxic effects by inducing apoptosis via activation of BAD and BCL-2, as well as through activation of mitogenic pathways.
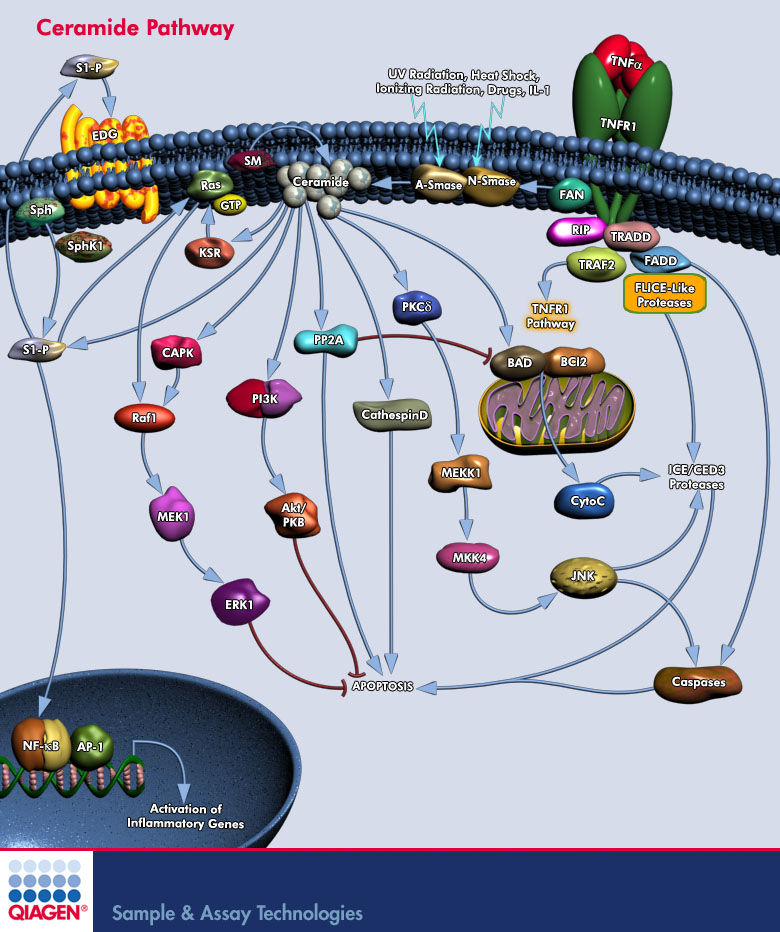
The Ceramide Pathway The SM (Sphingomyelin) pathway is an evolutionarily conserved stress response system linking diverse environmental stresses (Ultraviolet, Heat Shock, Oxidative Stress, and Ionizing Radiation) to cellular effector pathways. Ceramide is the second messenger in this system and can be generated either by hydrolysis of SM through SM-specific PLC (Phospholipase-C) termed SMases (Sphingomyelinases) or by de novo synthesis through the enzyme Ceramide Synthase (Ref.1). There are two classes of SMase, acidic (A-Smase) and neutral (N-Smase). Stress stimuli such as, TNF-Alpha (Tumor Necrosis Factor-Alpha), lipopolysaccharide, and some chemotherapy drugs such as doxorubicin also mediate apoptosis by generation of the lipid second messenger, Ceramide. A specific interaction between the FAN (Factor Associated with N-Smase activation) protein and TNF-Alpha associated proteins (TRADD, TRAFs and RIP) regulates Ceramide production by N-Smase, a step crucial in TNF signaling. Sphingolipids are integral components of eukaryotic cell membranes and their metabolisms generates and interconvert various metabolites including Ceramide, Sphingosine, and S-1P (Sphingosine-1 Phosphate), which affect cell cycle, apoptosis, and angiogenesis (Ref.2). S-1P activates the heterotrimeric orphan receptor EDG /S1PRs (Endothelial Differentiation, Sphingolipid GPCR) to stimulate SphK1 (S-1P Kinase) and to generate intracellular S-1P from Sph (Sphingosine). S-1P functions in an autocrine or paracrine fashion to stimulate the EDG /S1PRs (Endothelial Differentiation, Sphingolipid GPCR) that are present on the cell surface of the same or nearby cells. Coupling of EDG /S1PRs to diverse G-Proteins leads to activation of numerous downstream signalling pathways. TNF-Alpha and other cytokines stimulate SphK1 leading to the activation of the transcription factors NF-KappaB and AP-1 (Adaptor Protein-1), which is essential for the prevention of apoptosis. Either alone, or in combination with other signals, Ceramide, once generated, propagates the cellular stress response by coupling to effector systems. Different cells react differently to elevations in Ceramide: some cells launch the apoptotic program; others commit to terminal differentiation, or undergo cell cycle arrest, depending on the effector pathways activated (Ref.1). Well-defined targets are CAPK (Ceramide-Activated Protein Kinase), Ras , CAPP (Ceramide-Activated Protein Phosphatase), PP2A, and PKC-Delta (Protein Kinase-C-Delta). Ceramide binding to Raf1 leads to sequestration of Raf1 into inactive Ras-Raf1 complexes. In addition, the putative GEF (Guanine Nucleotide Exchange Factor), Vav, a potential activator of Ras and related proteins in hematopoietic cells, serve as a direct effector of Ceramide. Ceramide directly regulates KSR (Kinase Suppressor of Ras), PLA2 (Phospholipase-A2), Cathepsin-D, various PKC (Protein Kinase-C) isoforms, and c-Raf1 (Ref.8). Ceramide induce apoptosis by activating pro-death pathways through activation of JNK (c-Jun N-terminal kinase) /SAPK (Stress-Activated Protein Kinase), and by promoting dephosphorylation of the pro-survival protein BCL2, BAX and the pro-apoptotic protein BAD. The dephosphorylation of BCL2 is mediated by CAPP activity, whereas dephosphorylation of BAD by Ceramide is through activation of the KSR and subsequent activation of MEK1 (MAPK/ERK Kinase) pathway and MAPK (Mitogen-Activated Protein Kinase), leading to decreased PKB (Protein Kinase-B) activation (Ref.3). Ceramide also directly inhibits PI3K (Phosphoinositide-3 Kinase), together blocking Akt/PKB activity and BAD phosphorylation. Phosphorylated CAPK also activates Raf1, which then phosphorylates and activates the two dual specificity protein kinases, MAPK, ERK1/2(Extracellular Signal-Regulated Kinase), triggering the MAPK/ERK pathway. Consistent with this, stimulation of the SM pathway induces ERK activation and NF-KappaB (Nuclear Factor kappa-B) translocation. Activation of JNK by Ceramides regulates the transcription factor AP-1 (Activating Protein-1) and induces the expression of a novel monocyte/macrophage differentiation-dependent gene (Ref.4). Ceramide activates the SAPK cascade via direct activation of PKC-Delta (Protein Kinase-C). Upon activation by Ceramide, PKC-Delta forms a signaling complex with upstream components of the SAPK cascade, including MEKK1 (MAPK/ERK Kinase Kinase) and SEK1 (or MAP2K4). Ceramide also activates proteinases including the Caspases , especially Caspase3, (otherwise known as the ICE family (IL-3 Beta Converting Enzyme) of proteases such as CPP32) that mediate intracellular protein degradation (Ref.5). Ceramide also activates endonucleases that are responsible for DNA cleavage. A-Smase-released Ceramide binds directly to PLA2 and Cathepsin-D, which induce apoptosis (Ref.8).
The National Cancer Institute (NCI) is advancing research on BXQ-350 nanovesicle treatment of cancer to generate data needed for filing an investigative new drug (IND) application to the U.S. Food & Drug Administration (FDA), which is required prior to the start of clinical studies with the drug.
The FDA Office of Orphan Products Development grants orphan drug designation to novel drugs and biologics that are intended for the safe and effective treatment, diagnosis or prevention of rare diseases or disorders that affect fewer than 200,000 people in the United States.