For patients with activating mutations to the EGFR receptor kinase, small molecule specific inhibitors Iressa (gefintinib) or Tarceva (erlotinib) are administered as front-line treatments. But, non-small cell lung cancers develop resistance after about one year of treatment via a single recurrent missense mutation (T790M) to the tyrosine kinase. This is similar to resistance observed in patients with CML (Chronic Myeloid Leukemia) following treatment Gleevec (imatinib), which is mediated by a mutation to the Bcr-Abl transgene.
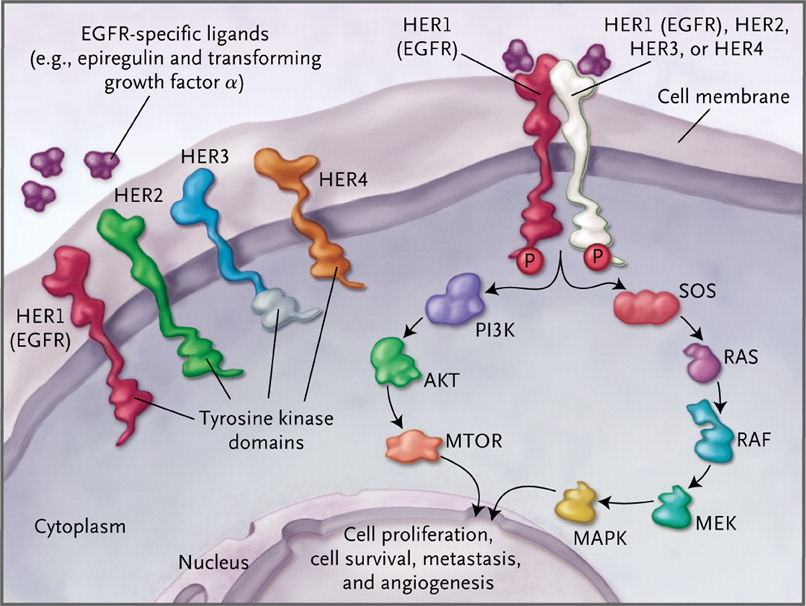
The EGFR is a critically important transmembrane receptor that triggers the ras signaling cascades, including MEK and Akt/PKB, which results in proliferation, and blocking of differentiation and apoptosis – see diagram from NEJM. Development of drugs to overcome the T970M mutation is very important.
Two such drugs are CO1686 (Clovis Oncology) and AZD9291 (AstraZeneca), which are designed to irreversibly block both the tyrosine kinase receptors of the activated EGFR mutation, and the T970M mutation – EGFRm+/T970M cancers. So, these could then be used in lieu of gefitinib or erlotinib. The chemical structures of all 4 compounds are shown, below:
In recent data reported at ASCO, CO1686 showed a 58% response rate in patients with the T970M mutation, however, 22% developed hyperglycemia requiring insulin treatment. AZD9291 showed a 50% response rate in Phase 1 studies. It will be interesting to see whether undesirable side effects also develop with AZD9291 given their different chemical structures.