Amgen reported encouraging data from a 189 patient phase 2 trial at this year’s ASCO meeting on blinatumomab in patients with Philadelphia Chromosome (9,22 translocation) negative relapsed/refractory B-precursor acute lymphoblastic leukemia (ALL) – see http://ecancer.org/conference/514-asco-2014/video/2913/blinatumomab-shown-to-be-beneficial-in-relapsed-refractory-b-precursor-acute-lymphoblastic-leukaemia.php.
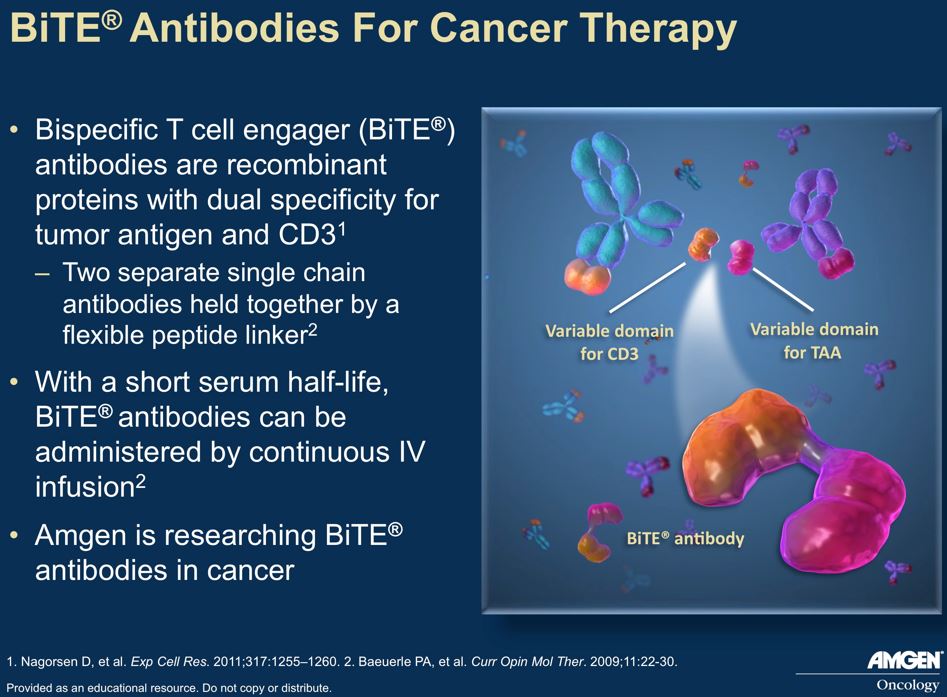
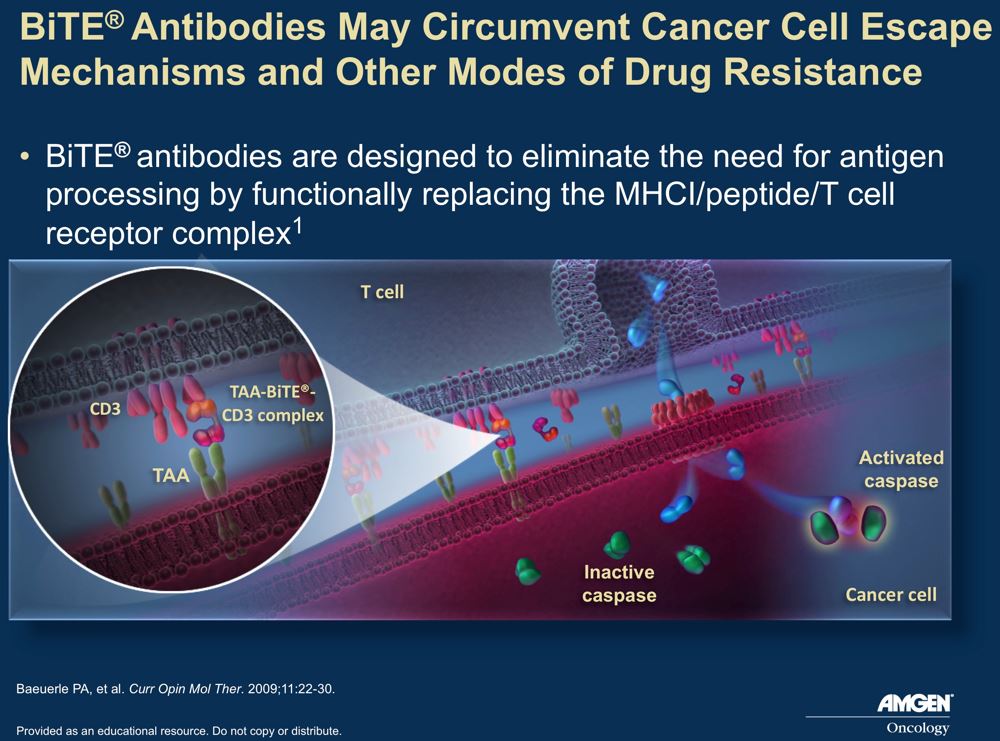
Amgen’s BiTE technology generates bi-specific antibodies designed to bring T-cells in proximity with cancer cells. In the case of blinatumumab, one variable region of the MAb targets CD3, a T-cell antigen required for T-cell activation, and the other targets CD19, which is expressed on the malignant B-cells – see diagram. The antibody brings the T cells and B cells in close proximity so that the T cells can engage and destroy the cancer, without the need for tumor associated antigen (TAA) processing and MHC Class I TAA recognition.
Fifty percent of patients with ALL are cured, by chemotherapy. The remainder will die within 2 years unless bone marrow transplant is performed. In the Phase 2 trial, 43% of patients that failed front-line chemotherapy had complete remission within 2 cycles. This therapy can extend the time required for BMT, without the need for additional toxic chemotherapy. Finding appropriate BMT donors is not easy, so, blinatumumab can serve as a less toxic “bridge” to BMT. Self-limited neurologic toxicity has been observed with blinatumumab, in a addition to cytokine release syndrome.
Today, Amgen reported that it received Breakthrough Therapy Designation from the FDA. The FDA states that Breakthrough Therapy Designation is intended to expedite the development and review of drugs for serious or life-threatening conditions. The criteria for Breakthrough Therapy Designation require preliminary clinical evidence that demonstrates the drug may have substantial improvement on at least one clinically significant endpoint over available therapy. A Breakthrough Therapy Designation conveys all of the fast-track program features, more intensive FDA guidance on an efficient drug development program, an organizational commitment involving senior managers, and eligibility for rolling review and priority review.