Hepatocellular carcinoma (HCC) is a primary cancer of the liver that occurs as a result of chronic liver disease, including cirrhosis and hepatitis B and C infections (Figure 1). Serum alpha-fetoprotien (AFP) levels are elevated early in the disease, and screening of patients with chronic liver disease for AFP has lead to earlier diagnosis of HCC. The test is 40-64% sensitive (the ability to detect disease when disease is truly present) because many HCCs do not produce AFP, but it is 75-91% specific (the ability to rule out disease when disease is truly absent) – an AFP of over 400 mg/mL is considered diagnostic.
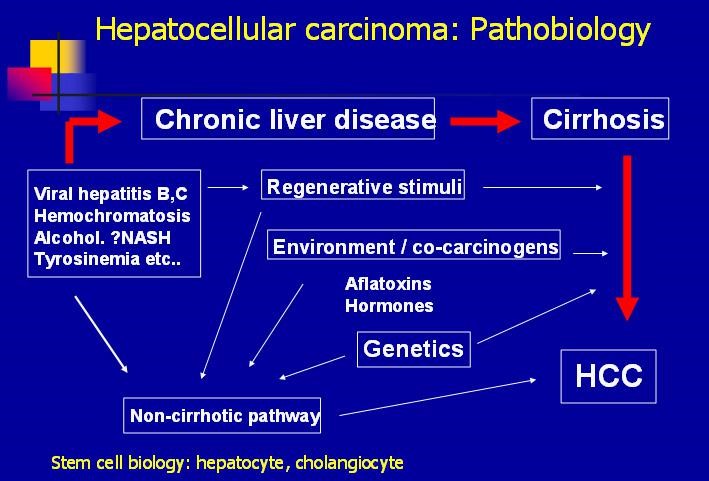
Figure 1. Pathobiology of hepatocellular carcinoma (HCC) – http://emedicine.medscape.com/article/197319-overview#a7
The incidence of HCC is in the rise, and the expected peak due to hepatitis C infection has yet to be reached:
In the United States, HCC, with its link to the hepatitis C epidemic, represents the fastest growing cause of cancer mortality overall and the second fastest growing cause of cancer deaths among women, according to data from the Surveillance Epidemiology and End Results (SEER) program.
Over the past 20 years, the incidence of HCC has more than doubled, from 2.6 to 5.2 per 100,000 population. Among African Americans, the increase has been even greater (ie, from 4.7 to 7.5 per 100,000 population overall and to 13.1 per 100,000 population among males). Mortality has similarly increased from 2.8 to 4.7 per 100,000 population over the past decade alone.
Treatment approach to HCC
The average age at diagnosis is 64 years; 74% of patients are men. Treatment includes resection and liver transplantation for patients with very early disease, to percutaneous ethanol injection and radiofrequency thermal ablation for early disease, and chemoembolization, chemotherapy, experimental therapy and palliative care (Figure 2).
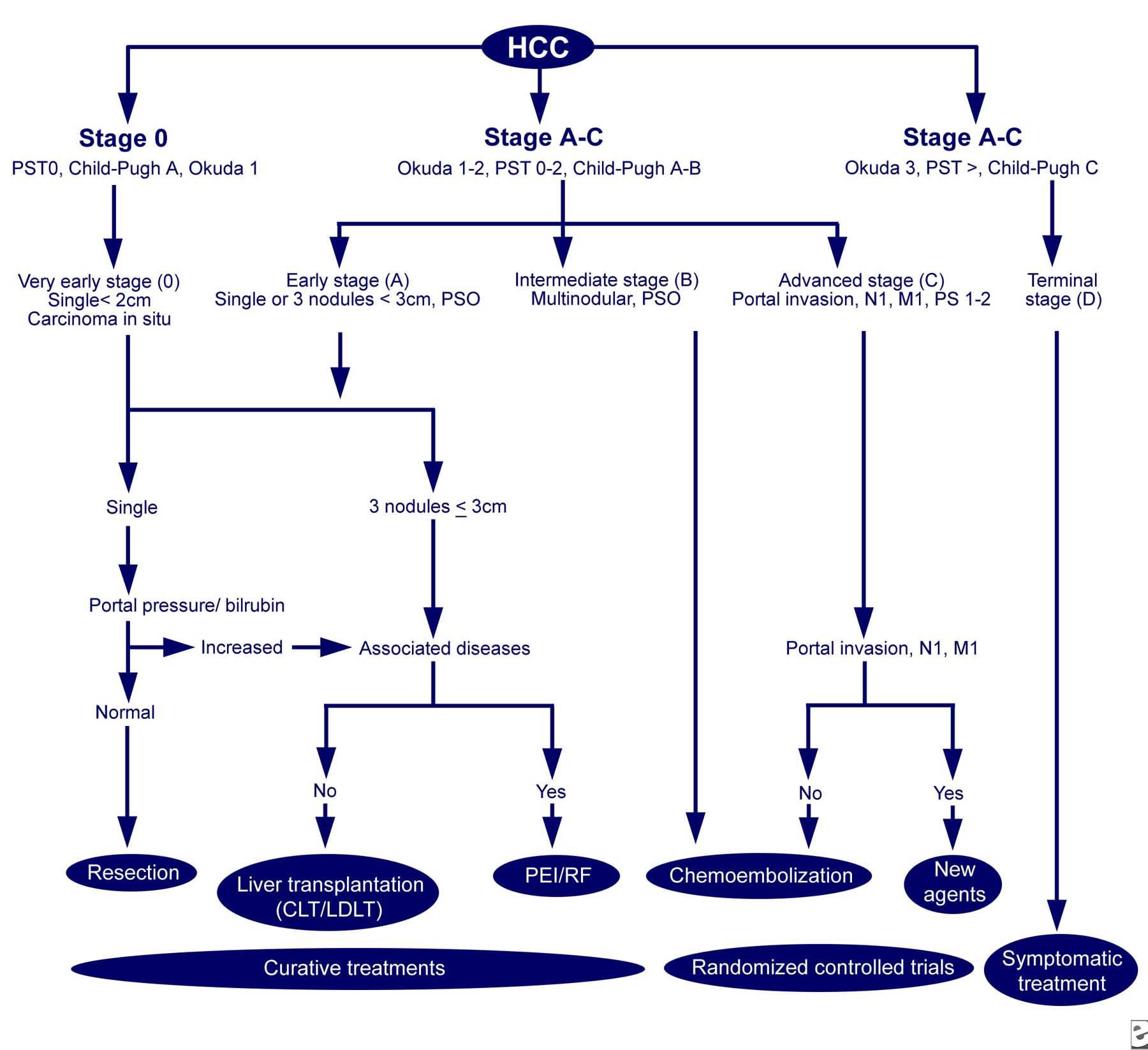
Figure 2. The Barcelona-Clinic Liver Cancer (BCLC) approach to hepatocellular carcinoma management. Adapted from Llovet JM, Fuster J, Bruix J, Barcelona-Clinic Liver Cancer Group. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. Feb 2004;10(2 Suppl 1):S115-20. http://emedicine.medscape.com/article/197319-workup#c8
Tyrosine Kinase Inhibitors for HCC
For patients with advanced disease, HCC does not respond well to systemic chemotherapy – doxorubicin, the most active chemotherapeutic agent, offers a 20-30% response rate and minimal impact on survival. The tyrosine kinase inhibitor, sorafenib (Nexavar), has been approved for the treatment of unresectable HCC.
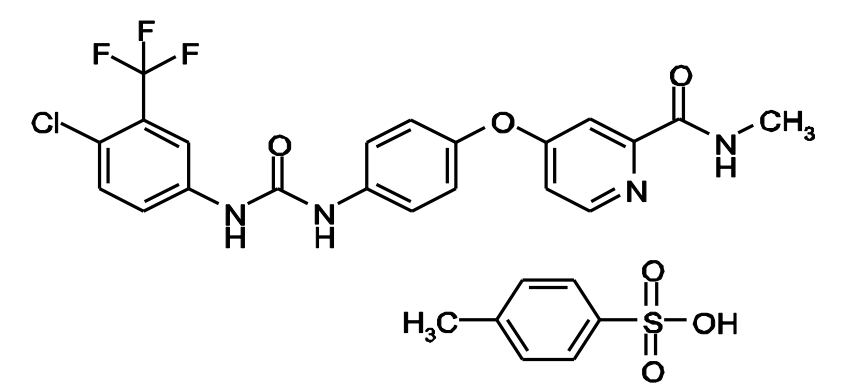
Figure 3. Chemical structure of sorafenib. http://labeling.bayerhealthcare.com/html/products/pi/Nexavar_PI.pdf
Sorafenib inhibits multiple intracellular (c-CRAF, BRAF and mutant BRAF) and cell surface kinases (KIT, FLT- 3, RET, RET/PTC, VEGFR-1, VEGFR- 2, VEGFR- 3, and PDGFR-ß). Several of these kinases are thought to be involved in tumor cell signaling, angiogenesis and apoptosis.
In a phase 3 study of 602 patients with unresectable HCC (randomized to sorafenib (400 mg twice daily; n = 299) and placebo (n = 303), statistically significant increases in progression free survival (5.5 months versus 2.8 months, respectively; hazard ratio 0.58; p < 0.000007), and overall survival (10.7 months versus 7.9 months, respectively; hazard ratio 0.69; p < 0.00058) were observed.
Regorafenib (Stivarga) is a multi-tyrosine kinase inhibitor that was approved for the treatment of patients with HCC who have been previously treated with sorafenib.
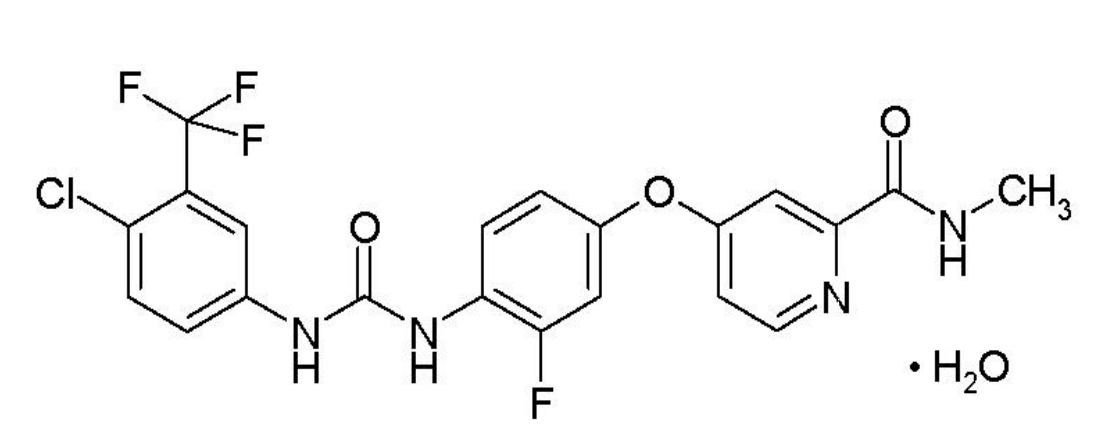
Figure 4. Chemical Structure of regorafenib. http://labeling.bayerhealthcare.com/html/products/pi/Stivarga_PI.pdf
Regorafenib is a small molecule inhibitor of multiple membrane-bound and intracellular kinases involved in normal cellular functions and in pathologic processes such as oncogenesis, tumor angiogenesis, metastasis and tumor immunity. In in vitro biochemical or cellular assays, regorafenib or its major human active metabolites M-2 and M-5 inhibited the activity of RET, VEGFR1, VEGFR2, VEGFR3, KIT, PDGFR-alpha, PDGFR-beta, FGFR1, FGFR2, TIE2, DDR2, TrkA, Eph2A, RAF-1, BRAF, BRAF V600E, SAPK2, PTK5, Abl and CSF1R at concentrations of regorafenib that have been achieved clinically.
A phase 3 study of patients with HCC who had disease progression following sorafenib was conducted – “REgorafenib after SORafenib in patients with hepatoCEllular carcinoma” (RESORCE). Patients were randomized 2:1 to regorafenib 60 mg per day (plus best supportive care, BSC; n = 379) or placebo + BSC (n = 194). Patients treated with regorafenib had a statistically significant increased overall survival compared with patients receiving placebo (10.6 months versus 7.8 months, respectively; hazard ration 0.63; p < 0.0001) and progression-free survival (3,1 months versus 1.5 months, respectively; hazard ratio 0.46 ; p < 0.0001).
Adverse events with multi-tyrosine kinase inhibitors
Dose interruptions for adverse events were required in 58.3% of patients receiving regorafenib and 48% of patients had their dose reduced. The most common adverse reactions requiring dose modification (interruption or dose reduction) were HFSR/PPES (hand and foot skin reactions, palmar-plantar erythrodysesthesia occurring in 20.6%), blood bilirubin increase (5.9%), fatigue (5.1%) and diarrhea (5.3%). Adverse reactions that resulted in treatment discontinuation were reported in 10.4% of STIVARGA-treated patients compared to 3.6% of patients who received placebo; the most common adverse reactions requiring discontinuation of STIVARGA were HFSR/PPES (1.9%) and AST increased (1.6%).
Twenty-one percent of patients with HCC taking sorafenib also experienced HFSR/PPES. In breast cancer studies of sorafenib, up to 60% of patients experienced HFSR. The incidence of HFSR is higher when sorafenib is combined with anti-angiogenic therapies.
HFSR/PPES is a common adverse event in patients taking multi-kinase inhibitors. It is distinct from HFS (hand-foot syndrome), which occurs with chemotherapeutic agents.
HFS presents with diffuse painful edema and redness of palms and soles. Whereas HFSR is dose-dependent and very characteristically localizes to areas of pressure or friction on the skin, such as on the heels, metatarsal heads, and areas of friction caused by shoes or manual labour. Lesions are sharply demarcated, erythematous, edematous, painful and very tender blisters that evolve into inflamed and painful skin adjacent to the calluses.
Because chemotherapeutic agents – taxanes, anthracyclines – cause HFS (hand-foot syndrome), and capecitabine also causes HFSR, the concomitant administration of multi-kinase inhibitors and chemotherapeutics must be performed with caution.

Figure 5. Hand-foot skin reaction and hand-foot syndrome. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3233284/ (i) Childress J, Lokich J. Cutaneous hand and foot toxicity associated with cancer chemotherapy. Am J Clin Oncol 2003;26:435–436, with permission. (ii) Photographs reprinted from Autier J, Escudier B, Wechsler J et al. Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermatol 2008;144:886–892 (multikinase inhibitors). Copyright © 2008 American Medical Association. All rights reserved. (iii) Lassere Y, Hoff P. Management of hand-foot syndrome in patients treated with capecitabine (Xeloda). Eur J Oncol Nurs 2004;8(suppl 1):S31–S40 (anthracyclines/antimetabolites), with permission, and photograph courtesy of Mario Lacouture, M.D. (taxane).

HCC REG
Regorafenib approved for HCC
The best article to share with cancer patients, may patients are given healing. Greetings from us, CancerOZ