The FDA granted Breakthrough Therapy Designation to Sacituzumab govetican (IMMU-132) for treatment of triple-negative breast cancer (TNBC). A diagnosis of triple negative breast cancer means that the three most common types of receptors known to fuel most breast cancer growth–estrogen, progesterone, and the HER-2/neu gene– are not present in the cancer tumor. This means that the breast cancer cells have tested negative for hormone epidermal growth factor receptor 2 (HER-2), estrogen receptors (ER), and progesterone receptors (PR). Since the tumor cells lack the necessary receptors, common treatments likehormone therapy and drugs that target estrogen, progesterone, and HER-2 are ineffective.
Trop-2, a cell surface protein is widely expressed in a variety of epithelial cancers (Figure 1). This cell surface protein is presents in 90% of triple- negative breast cancer patients. Trop-1 and Trop-2 are homologous to serum IGF-11-binding proteins. IGF-binding proteins increase the half-life of insulin-like growth factor, potentiate their effect on cell proliferation, localize IGF to specific receptors on the cell surface, and block the activity of IGF by inhibiting cell surface binding. For example, p53 induces the expression of IGF-BP, which blocks Akt/PKB mediated inactivation of pro-apoptotic Bad and IkB kinase, thereby liberating NF-kB to induce anti-apoptotic Bcl-2 and Inhibitors of Apoptosis. Interestingly, Trop-2, while homologous with IGF-11 BP’s transduces signals that result in the activation of the MAPK pathway (see Figure 2).
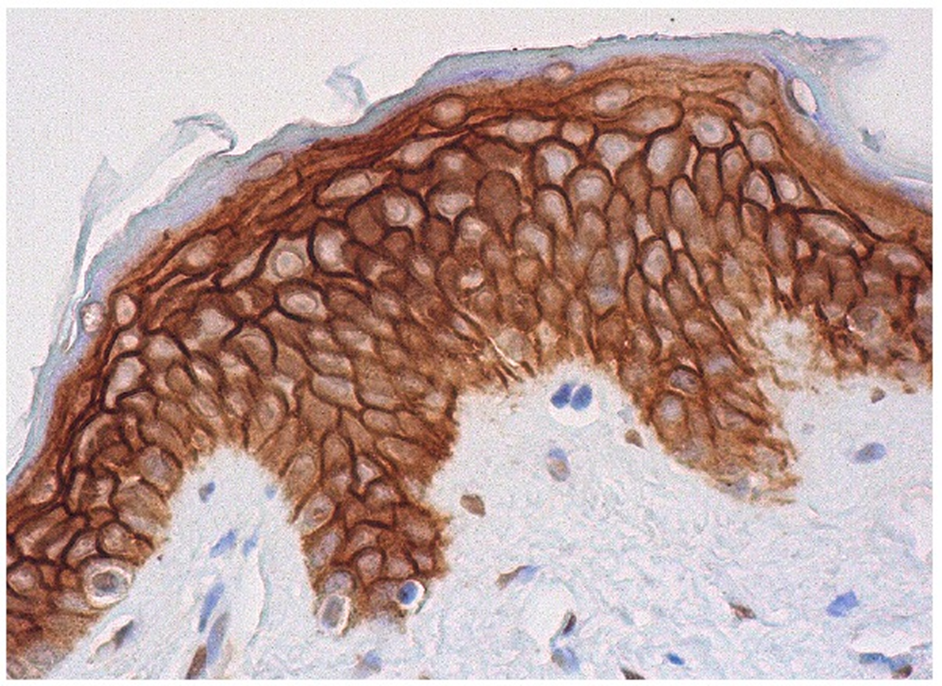
Immunoperoxidase staining of formalin fixed, paraffin-embedded human skin tissue showing membrane and cytoplasmic staining of epidermal cells. http://www.scbt.com/datasheet-376746-trop-2-b-9-antibody.html
Trop-2 is a signal-transducing receptor that results in transient elevated intracellular Ca++ levels. In epithelial ovarian cancer (EOC), Trop-2 protein overexpression correlates with an aggressive malignant phenotype.
Trop-2 expression is invariably upregulated in tumours, regardless of baseline expression in normal tissues, which suggests a corresponding selective advantage. Overexpression of wild-type Trop-2 was shown to be necessary and sufficient to drive cancer growth in a widely invariant manner across cell type and species. Upregulation of Trop-2 was shown to quantitatively stimulate tumour growth, as proportional to expression levels in vivo, and tumour cell growth was abrogated by somatic knockdown of Trop-2 expression. Above-baseline expression of wild-type Trop-2 is a key driver of human cancer growth.
In addition to transducing proliferative signals through the MAPK signaling pathway, it also mediates signaling for integrin binding.
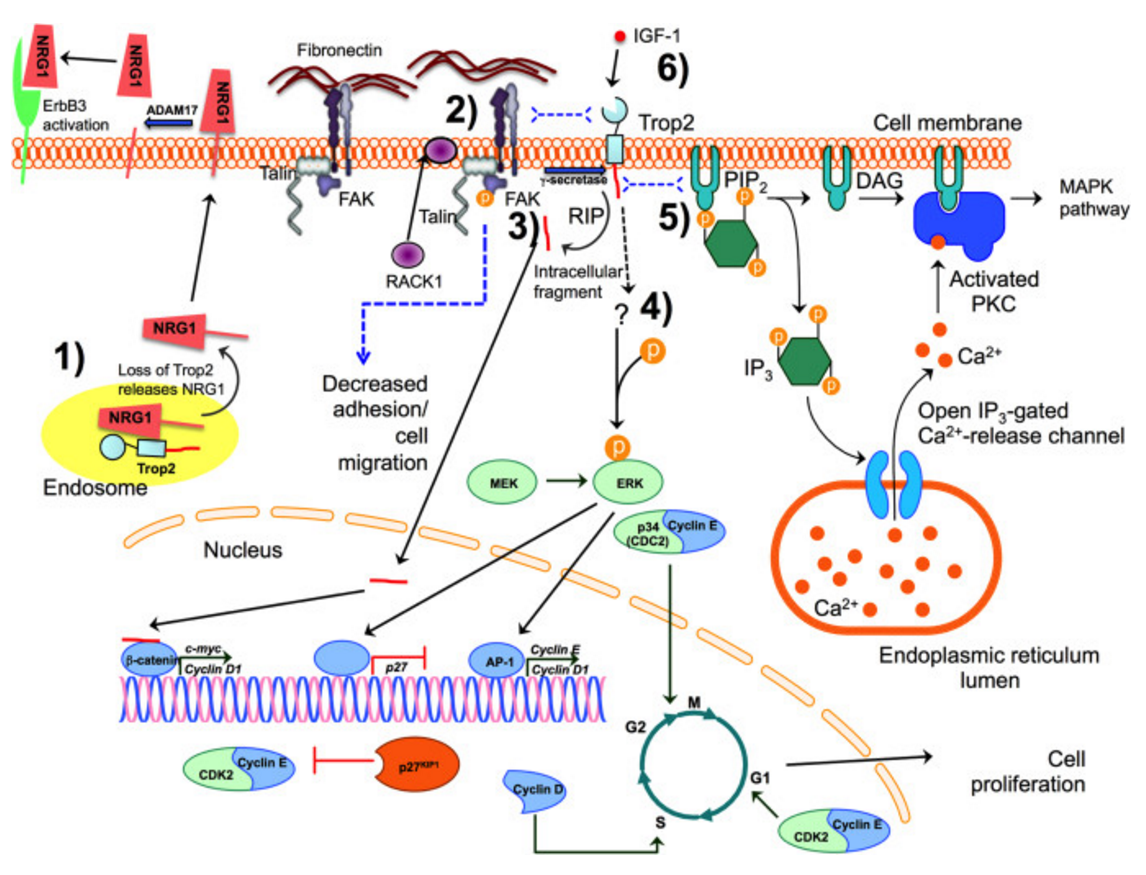
Proposed TROP2 signaling pathways. TROP2 is a membrane-bound protein with a large extracellular domain, a single transmembrane region, and a short intracellular tail, although it is also found in the cytosol. TROP2 may exert its effects on cell proliferation, adhesion, and migration by activating several different intracellular signaling pathways. (1) Membrane-bound (not shown) or intracellular TROP2 binds to and likely sequesters neuregulin 1 (NRG1) and regulates the trafficking of NRG1 to or from the cell surface. Upon loss of Trop2 in some cancers, NRG1 membrane-bound precursors accumulate at the cell surface, where they can be cleaved by ADAM17 releasing the NRG1 ectodomain, which can then bind to and activate the EGF family receptor, ErbB3 (Zhang et al., 2014). (2) TROP2 promotes the localization of RACK1 from the cytoplasm to the membrane, bringing it in closer proximity to integrin β-1, resulting in a decrease in fibronectin binding. TROP2 associates with integrin β-1 and talin, bringing the integrin α5β1/talin complex to the leading edge of the cell and also increases the phosphorylation of FAK. This leads to increased cell motility and decreased cell adhesion (Trerotola et al., 2013b). (3) TROP2 undergoes regulated intramembrane proteolysis (RIP) at two cleavage sites mediated by the γ-secretase complex and results in a large extracellular fragment and a short intracellular fragment. The intracellular fragment can enter the nucleus, where it binds to the β-catenin transcription factor, increasing the expression of cyclin D1 and c-myc (Stoyanova et al., 2012), promoting cell proliferation and self-renewal. (4) TROP2 signals via the MAPK pathway, by regulating the level and phosphorylation of ERK (Cubas et al., 2010; Guerra et al., 2012; Lin et al., 2012; McDougall et al., 2013). TROP2 promotes ERK-mediated activity of the AP-1 transcription factor, increasing the expression of cyclin D1, cyclin E, and cyclin-dependent kinases (CDK), promoting cell cycle progression and cell proliferation. TROP2-mediated ERK activity also decreases cell cycle inhibitor p27/kip1, which inhibits the activity of the cyclin E/CDK2 complex (Cubas et al., 2010; Liu et al., 2013b). (5) TROP2 activation increases intracellular calcium concentrations (Ripani et al., 1998). The intracellular tail of Trop2 contains a putative PIP2 binding site, which is the likely pathway leading to increased calcium. Hydrolysis of PIP2 results in the production of DAG and IP3. IP3 binds to the IP3 receptor (IP3R), resulting in release of calcium from intracellular stores in the endoplasmic reticulum. DAG activates the PKC pathway, which in turn, activates the MAPK pathway, possibly providing a mechanism by which TROP2 regulates ERK activity. In addition, the intracellular tail of TROP2 contains a serine that can be phosphorylated by PKC. However, the role of TROP2 phosphorylation is yet to be determined. (6) TROP2 can bind to and likely sequester IGF-I, preventing it from activating the IGF-1R and therefore inhibiting IGF-I/IGF1-R signaling pathways (Lin et al., 2012). Dotted lines indicate putative binding or signaling pathways. Image not to scale. https://www.researchgate.net/figure/269722966_fig2_Figure-3-Proposed-TROP2-signaling-pathways-TROP2-is-a-membrane-bound-protein-with-a
Sacituzumab govetican is a humanized IgG antibody targeted against Trop-2 conjugated to SN-38, the active metabolite of irinotecan, which is a topoisomerase inhibitor (see Figure 3).
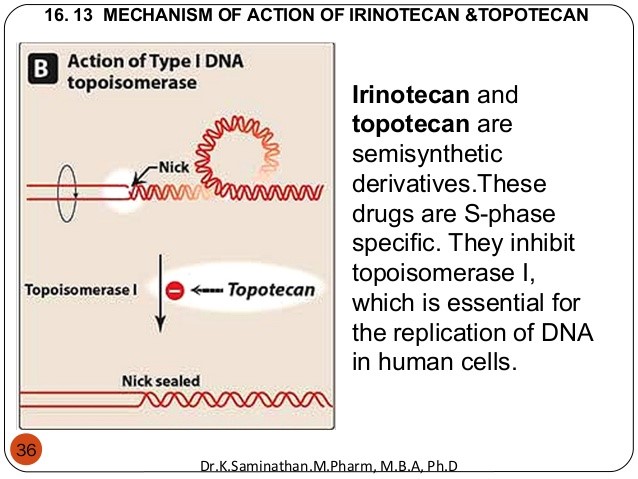
Topoisomerase 1 is critical for DNA uncoiling during replication; its blockade results in DNA single strand breaks (see Figure 4).
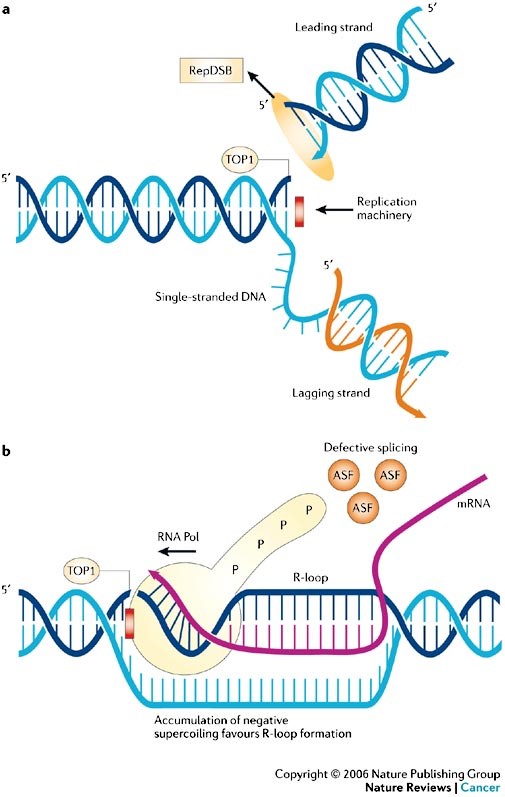
Human DNA topoisomerase I is an essential enzyme that relaxes DNA supercoiling during replication and transcription. Top1 generates DNA single-strand breaks that allow rotation of the cleaved strand around the double helix axis. Top1 also religates the cleaved strand to reestablish intact duplex DNA. The Top1-DNA intermediates, known as cleavage complexes, are transient and at low levels under normal circumstances. However, treatment with Top1 inhibitors, such as the camptothecins, stabilize the cleavable complexes, prevent DNA religation and induce lethal DNA strand breaks. Cancer cells are selectively sensitive to the generation of these DNA lesions. http://america.pink/topoisomerase-inhibitor_4493616.html
Phase II results (Figure 5) were presented at the 2015 San Antonio Breast Cancer Symposium. In the study, patients with metastatic TNBC received either 8 or 10 mg/kg of sacituzumab govitecan intravenously on days 1 and 8 of 21-day repeated cycles. Patients received a median number of 8 doses (range, 1-37). Response was measured by RECIST1.1 criteria.
- At the data cutoff, 60 patients who had progressed on at least 2 prior treatments, including a taxane, had received sacituzumab govitecan. Seventy percent of these patients had an ECOG performance status of 1, with the remaining 30% having an ECOG performance of 0. The median age was 54 years (range, 31-80 years).
- The median number of prior therapies was 5 (range, 2-12). Prior therapies received included cyclophosphamide (93%), doxorubicin (83%), carboplatin (57%), gemcitabine (50%), capecitabine (43%), eribulin (38%), cisplatin (25%), vinorelbine (17%), and bevacizumab (13%).
- Eighteen of 58 evaluable patients had a response, for an overall response rate (ORR) of 31%. There were 2 complete responses and 16 partial responses (PR). The clinical benefit ratio (CR + PR + SD) was 53% at ≥4 months and 45% at ≥6 months.
- The median progression-free survival (PFS) was 6.0 months (95% CI, 4.1-9.4 months) and the overall survival (OS) data were not yet mature, with 83% of patients still alive. The 10 mg/kg regimen was the dose selected for future clinical development.
- The most common all-grade adverse events (AEs) in the 10 mg/kg arm were diarrhea (27%), nausea (27%), neutropenia (23%), vomiting (22%), alopecia (18%), anemia (17%), fatigue (17%), constipation (13%), rash (13%), and abdominal pain (10%).
- The most frequently reported grade ≥3 AEs in the 10 mg/kg cohort were neutropenia (15%), leukopenia (8%), diarrhea (5%), fatigue (3%), dyspnea (3%), febrile neutropenia (2%), and anemia (2%).