Two companies announced news last week regarding their efforts to treat cancer by administering treatments that become activated by physiological conditions specific to the tumor microenvironment. Threshold Pharmaceuticals announced data from Phase III clinical trials of evophosphamide (TH-302), and BioAtla announced an antibody development deal with Pfizer.
Tumor Hypoxia – Threshold
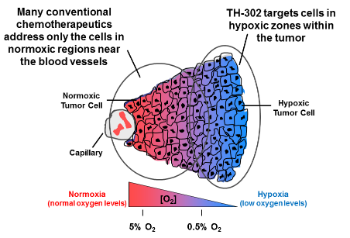
Regions of tumor hypoxia, or low-oxygen conditions, is a common feature of the microenvironment of many solid tumors. The network of blood vessels supplying solid tumors is known to be highly irregular and disordered, failing to deliver sufficient oxygen and nutrients to the rapidly dividing cancer cells. Compromised oxygen delivery results in the development of regions of tumors that are chronically and transiently hypoxic. Hypoxia is associated with increased tumor resistance to chemotherapy and radiation treatment. Many conventional reactive chemotherapies are not able to penetrate into the hypoxic zones that are located at a distance from the blood vessels.
Moreover, chronic hypoxia limits cancer cell proliferation, rendering the quiescent tumor cells in the hypoxic region of the tumor less susceptible to conventional antiproliferative agents, which typically target actively dividing cells in close proximity to the blood vessels. Under conditions of cell hypoxia, genomic instability can lead to tumor cell variants that can survive in an oxygen depleted environment through clonal selection and expansion. This clonal expansion leads to tumor progression, metastasis, acquired resistance to chemotherapy, and treatment failure, ultimately resulting in compromised clinical outcomes.
Threshold is engaged in the discovery and development of drugs that target tumor hypoxia, a common characteristic of the tumor microenvironment that is associated with tumor progression, metastasis, resistance to radiotherapy and standard chemotherapy, and ultimately treatment failure.
Evophosphamide (a bromo-analog of ifosfamide, a DNA alkylating agent) is, a prodrug that is activated only under hypoxic conditions commonly found in the tumor microenvironment. Within regions of tumor hypoxia, evofosfamide releases bromo isophosphoramide mustard (Br-IPM), a potent DNA alkylating agent. Br-IPM kills tumor cells by forming DNA crosslinks, rendering cells unable to replicate their DNA and divide as well as interfering with the transcription of DNA to make essential proteins. Once activated in hypoxic tissues, Br-IPM can also diffuse into surrounding oxygenated regions of the tumor and kill cells there via a “bystander effect.” Because of its preferential activation in the targeted hypoxic regions of solid tumors, evofosfamide may be less likely to produce broad systemic toxicity seen with untargeted cytotoxic chemotherapies.
Data from Phase III studies in pancreatic cancer and soft tissue sarcoma were disappointing:
- In the Phase 3 MAESTRO study, patients with previously untreated, locally advanced unresectable or metastatic pancreatic adenocarcinoma treated with evofosfamide in combination with gemcitabine did not demonstrate a statistically significant improvement in overall survival (OS) compared with gemcitabine plus placebo (hazard ratio [HR]: 0.84; 95% confidence interval [CI]: 0.71 – 1.01; p=0.0589).
- In the Phase 3 TH-CR-406/SARC021 study being conducted in collaboration with the Sarcoma Alliance for Research through Collaboration (SARC), patients with locally advanced unresectable or metastatic soft tissue sarcoma treated with evofosfamide in combination with doxorubicin did not demonstrate a statistically significant improvement in OS compared with doxorubicin alone (HR: 1.06; 95% CI: 0.88 – 1.29).
The company is pursuing additional products to exploit the tumor microenvironment:
- Tarloxotinib bromide (tarloxotinib – TH-4000), a clinical-stage Hypoxia-Activated Prodrug (HAP) that is designed to release an irreversible, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) under hypoxic conditions
- [18F]-HX4 [flortanidazole (18F)] is an investigational Positron Emission Tomography (PET) imaging agent for hypoxia developed by Siemens Healthcare Molecular Imaging (and acquired by Theshold in March 2013) to potentially identify and quantify the degree of hypoxia in tumors in vivo. PET is a non-invasive nuclear medical imaging technique that produces three-dimensional images of certain functional processes in the entire body or selected organs and tissues. [18F]-HX4 contains a short-lived radioisotope, 18F, that can be detected in a PET scanner. PET imaging is used to help physicians diagnose and treat cancer and is routinely performed in cancer treatment centers globally. [18F]-HX4 has a 2-nitroimidazole “trigger” that is designed to be activated under the extreme hypoxic conditions generally found in tumors but not typically in normal healthy tissue, therefore it will accumulate more in these hypoxic regions. Clinical data has demonstrated the potential of [18F]-HX4 to quantify the degree of hypoxia within different tumors.
Conditionally Active Biologics – Bioatla
Bioatla develops (or “evolves”) antibodies that become activated by physiologic conditions that are present in the tumor microenvironment, including:
- Temperature
- pH
- Osmotic pressure
- Osmolality
- Electrolyte or ion concentration (e.g. sodium, potassium, calcium, iron, etc)
- Hydrophobicity
- Oxidation
- Other physiological microenvironment conditions
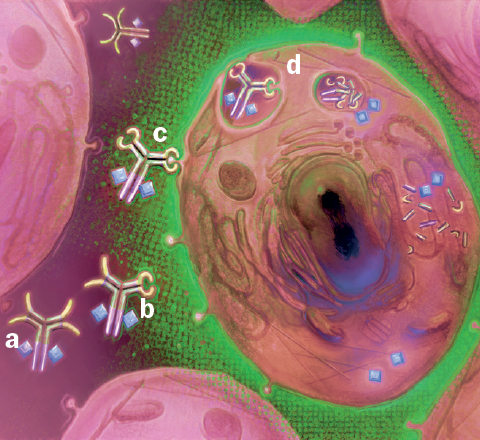
CAB mode of action as applied to improved cancer therapies. CAB-ADC passes benignly through normal tissue (a) until it encounters a tumor cell’s microenvironment (green). This activates the CAB’s binding ability (b) and the CAB binds to the tumor cell (c). Following internalization (d) the CAB releases its ADC payload, selectively killing the tumor cell.
The goal is to develop molecules with a higher therapeutic index, that is, with more activity against cancers with less side-effects that arise from targeting of normal tissues. This will increase safety by reducing “on-target toxicity,” namely drugs correctly binding their target but in an undesired part of the body, resulting in undesirable toxicity. Conditionally activated proteins can be engineered to function only in the correct, desired location in the body, minimizing such risks.
Pfizer entered into an agreement with BioAtla potentially worth more than $1 billion to develop a new class of antibody therapeutics for the treatment of cancer, BioAtla announced. The companies plan to use BioAtla’s Conditionally Active Biologic (CAB) platform in combination with Pfizer’s antibody drug conjugate (ADC) payloads.
What Are Antibody-Drug Conjugates?
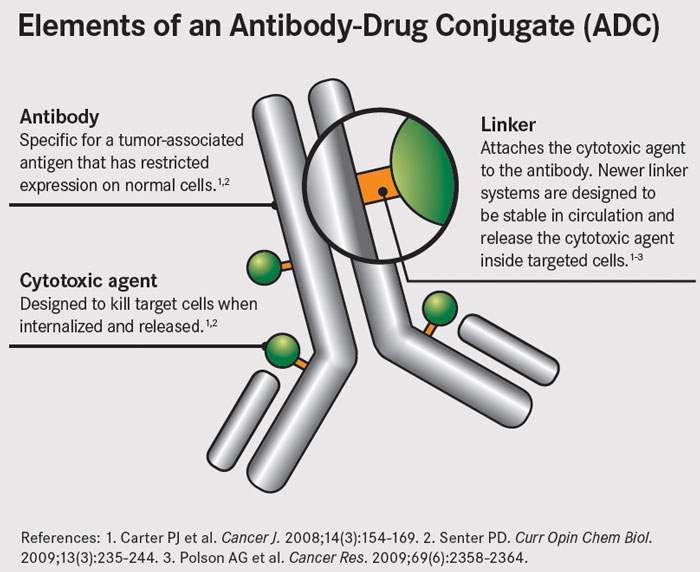
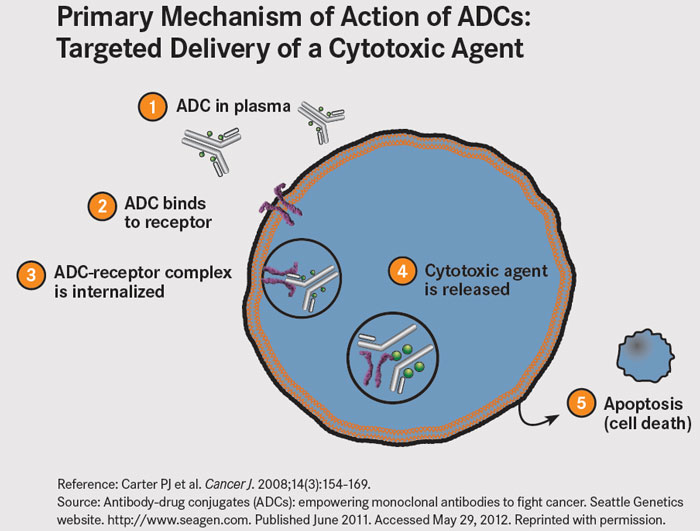
ADCs are therapeutic agents designed to target the delivery of chemotherapy to tumor cells. ADCs link cytotoxic agents to monoclonal antibodies that bind to tumor cell-specific antigens or to antigens that are overexpressed on the surface of tumor cells. The antibody acts as a sort of GPS system, and the theory is that this should increase delivery of potent cell-killing drugs to the tumor, while reducing the exposure of normal cells.
ADCs are made up of three parts: a monoclonal antibody, a cytotoxic drug, and a linker that joins the two together. The antibody guides the ADC to target tumor cells, where it binds to cell surface antigens. The ADC is then taken into the cell and the cytotoxic drug is released to perform its cell-killing function.