Thioureidobutyronitrile (Kevetrin) is an intravenous drug currently in a Phase 1 study in patients with advanced solid malignancies. It acts by promoting apoptosis in several ways:
Kevetrin induces transcription-independent p53 mediated apoptosis. Kevetrin enhanced the phosphorylation of MDM2. Phosphorylation of MDM2 alters the E3 ligase processivity. Stable monoubiquitinated form of wild type p53, accumulates in the cytoplasm and interacts with BAK or BAX proteins in mitochondria to induce apoptosis Thus Kevetrin activates both transcription dependent and transcription independent pathways to promote apoptosis.
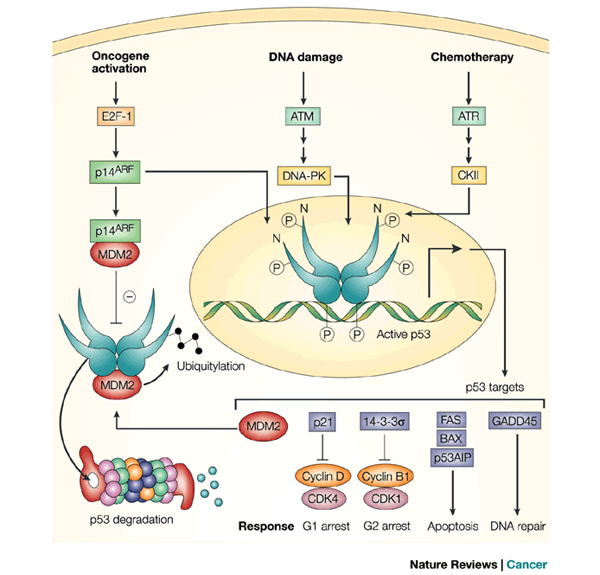
Approximately 50% of all tumor types carry a p53 mutation. These mutations are typically seen in the DNA binding domain, which affects transcriptional activity and its overall cellular activities. The elevated expression of p53 leads to greater stability, which also induces the regulatory protein Mdm2. Mdm2 is a proto-oncogene that is amplified in approximately 7% of cancers and is frequently seen in soft tissue tumors. A proto-oncogene is a gene that becomes an oncogene, a gene that has the potential to cause cancer, through mutations or an increase in expression. The combination of a p53 mutation with overexpression of Mdm2 results in a worse prognosis for a patient, as compared to a patient with only the mutation or the overexpression. Mdm2 protein has the activity of an ubiquitin ligase, which allows for the targeted degradation of its substrates, including p53. In addition to marking p53 for degradation, Mdm2 also binds to p53 and transports it out of the nucleus into the cytoplasm for degradation. The phosphorylation of p53 affects Mdm2’s ability to target p53 for degradation. When stress or DNA damage occurs in the body and the phosphorylation of p53 occurs on multiple sites, Mdm2 does not associate with p53. Researchers have recently discovered Mdm2 in human tumors and these scientists hypothesize that Mdm2 plays a role in tumorigenesis, with or without p53. The relationship between p53 and Mdm2 has been shown to be vital to the normal functioning of the human cell, and also has other implications in cancer. Additionally, scientists have found that this relationship is an important part of a number of complex cellular signaling cascade pathways, including Ras, β-catenin, myc, Rb, and many more. The complexity of the p53-Mdm2 link illustrates the importance of this signaling pathway and indicates it is a viable therapeutic target. Researchers are studying this signaling relationship in order to design targeted drugs and therapies for cancer, as well as many other diseases. http://www.cancer-biomarkers.com/2012_08_01_archive.html
Mechanism of action studies showed that Kevetrin strongly induced apoptosis by activation of Caspase 3 and cleavage of PARP. Kevetrin induced phosphorylation of p53 at Ser15 leading to a reduced interaction between p53 and MDM2, an ubiquitin ligase for p53 that plays a central role in p53 stability. Stabilized wild type p53 induced apoptosis by inducing the expression of PUMA. In addition, Kevetrin increased expression of p53 target genes such as p21 (Waf1), an inhibitor of cell cycle progression.
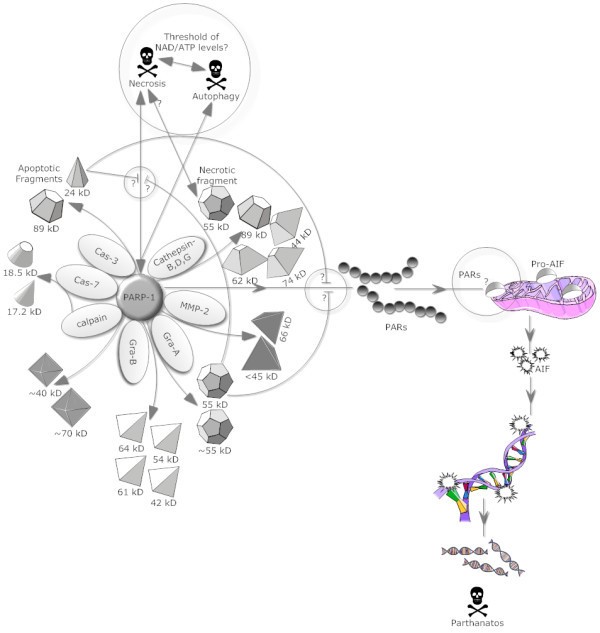
PARP-1 interactions with cell death proteases. PARP-1 cleavage by various suicidal proteases like caspases, calpain, cathepsins and granzymes liberates fragments with specific molecular weights and are shown in this schematic representation. Importantly, 21-kD and 55-kD PARP-1 fragments generated by caspase 3/7 and Gra-A respectively function as an inhibitor of PARP-1 activity and might also play important roles in reducing necrosis and/or parthanatos. Further, PARP-1 and PARP-1 fragment’s involvement in various forms of cell death e.g. autophagy, necrosis and parthanatos are also indicated. http://www.biosignaling.com/content/8/1/31


Kevetrin has been designated as an orphan drug as of 7.14.15 for the treatment of ovarian cancer.
http://www.accessdata.fda.gov/scripts/opdlisting/oopd/OOPD_Results_2.cfm
Here is an update:
http://cellceutix.com/wp-content/uploads/2015/05/ASCO-2015-Poster-.-FINAL-1.pdf
Thank you Joseph! Keep up the good work!
Excellent questions – I have written a book about developing new medical breakthroughs, obtaining FDA and Europen approval, and getting them on the market – see http://www.innovationbreakdownbook.com/. To asnwer your questions more specifically – (1) Kevetrin is in Phase 1 – if the data are favorable, I believe that the quickest time to FDA approval is 4 years; (2) yes, approval routes in other countries are faster, however, for small US based companies, it is advisable to aim for the US market initially; and (3) the FDA’s prime concern is risk with respect to the unknown – the status quo is known and accepted…no risk maintaining it. The FDA is loath to be on the cutting edge, rather, they sit back and wait for data to be compelling enough for them to take the risk of approving it. Sadly, in my experience, the FDA is not in the business of promoting health, rather, it is all about risk, and new things are risky…read my book and you’ll understand.
Joseph Gulfo, I was hoping you could perhaps answer some questions I have.
1. At this pace how long before a drug like Kevetrin could get FDA approval?
2. Would it be a faster pathway to approval if Kevetrin were to go through an approval process in another country in Europe?
3. Lastly, and more importantly, why isnt there any government agency pushing for the development of Kevetrin with so many people suffering?
I know this is alot but any feed back would be very much appreciated. Cancer really sucks so thank you for sharing this information and helping to get the word out.
Updated info on Kevetrin released today.
Ovarian Cancer Response -Potential Major Breakthrough in Oncology
View it in your browser.
Cellceutix Reports Spleen Lesion ‘Disappears’ in Patient with Metastatic Stage 4 Ovarian Cancer in Clinical Trial of Anti-Cancer Drug Kevetrin
Ovarian Cancer Response – Potential Major Breakthrough in Oncology
BEVERLY, MA–(Marketwired – Jan 20, 2015) – Cellceutix Corporation (OTC: CTIX) (the “Company”), a clinical stage biopharmaceutical company developing innovative therapies with oncology, dermatology and antimicrobial applications, is pleased to report the near complete disappearance of a metastatic lesion in the spleen of a Stage 4 ovarian cancer patient who was enrolled in the Company’s Phase 1 clinical trial of anti-cancer drug candidate Kevetrin™ being conducted at Harvard Cancer Center’s Dana-Farber Cancer Institute and Beth Israel Deaconess Medical Center. According to information supplied by the hospital, the patient, who successfully completed three Kevetrin 3-dose cycles before discontinuing the trial, experienced increased energy, while scans showed a reduction in the amount of peritoneal fluid (ascites) during treatment with Kevetrin. Subsequent to the second and third Kevetrin cycles, scans showed the spleen lesion to be essentially undetectable and the patient’s disease to be clinically stable.
With the completion of the ninth cohort, and commencement of tenth cohort at 450 mg/m2, the hospital has continued research to determine the effect of Kevetrin on p21, the key biomarker tightly controlled by the tumor suppressor protein p53. p53 is often referred to as the “Guardian Angel Gene” because of its crucial role in controlling cell mutations. In nearly all cancers, p53 is deficient or mutated, thus failing to perform its role as a master cell regulator, which exacerbates tumor progression and metastasis. As such, a drug to reactivate p53 to its normal state is a prime target for a next generation cancer therapy. Because p21 is a recognized downstream target of activated p53, increased levels of p21 in peripheral blood cells suggest that Kevetrin is having an impact on returning p53 to its effectiveness as a tumor suppressor.
Research conducted by the hospital evaluating p21 expression in earlier cohorts, for which the level of exposure to Kevetrin was substantially lower, showed that about 50 percent of the patients demonstrated at least a 10% increase in p21. The data supported the hypothesis that enhanced p21 is dose related, meaning that as the amount of Kevetrin increased, so did the p21 expression. Hospital scientists are now running samples examining the effect of Kevetrin on p21 at higher dosing levels in more recent cohorts and Cellceutix hopes to disclose the results shortly.
Additionally, the Principal Investigators for the Kevetrin trial have requested that the Company start preparing a presentation to be used for an article on the safety and pharmacological effect of Kevetrin on multiple cancer lines as demonstrated in the clinical trial. Cellceutix interprets this recommendation as a very optimistic sign regarding anticipated outcomes for the trial and clinical advancement of Kevetrin.
“I can’t overexpress the excitement at Cellceutix regarding Kevetrin or the significance of a metastatic lesion disappearing in a late-stage ovarian cancer patient,” commented Leo Ehrlich, Chief Executive Officer at Cellceutix. “We don’t know of any other company, regardless of specialization, albeit small molecule, immunotherapy or other, that has published an effect like that in such a hard-to-treat disease like metastatic ovarian cancer during a Phase 1 safety trial. The idea that a stage 4 ovarian cancer patient’s disease was clinically stabilized, although her CA-125 count was increased in the third month, is remarkable. The patients in our trial are incredibly sick, have often run the gamut of approved treatments and subject to constant therapy modification to address the greatest area of need at the given moment. That’s an everyday practice in oncology, especially when a drug regimen, such as the strict protocol with the Kevetrin trial where dosing levels and intervals absolutely cannot be changed. We are not privy to the minutiae underscoring any physician’s decisions in a trial, but we interpret the stabilization of the cancer as allotting the physician an opportunity to modify treatment to improve the patient’s quality of life, an opportunity that potentially may have not been there without Kevetrin. With that in mind, we are analyzing the collective preliminary data from the trial to date showing a strong safety profile for Kevetrin, an effect on p53 and data seeming to indicate that ovarian, pancreatic and other FDA designated orphan cancers would be ideal mid-stage clinical trial targets as we plan to move forward. Considering what has been shown recently in the Kevetrin trial and the recently completed Phase 2b trial of Brilacidin for ABSSSI, we believe that we have an incredibly strong franchise headlined by one of the most promising cancer drugs and one of the best antibiotics in development today.”
About Kevetrin:
As a completely new class of chemistry in medicine, Kevetrin has significant potential to be a major breakthrough in the treatment of solid tumors. Mechanism of action studies showed Kevetrin’s unique ability to affect both wild and mutant types of p53 (often referred to as the “Guardian Angel Gene” or the “Guardian Angel of the Human Genome”) and that Kevetrin strongly induced apoptosis (cell death), characterized by activation of Caspase 3 and cleavage of PARP. Activation of p53 also induced apoptosis by inducing the expression of p53 target gene PUMA. p53 is an important tumor suppressor that acts to restrict proliferation by inducing cell cycle checkpoints, apoptosis, or cellular senescence.
In more than 50 percent of all human carcinomas, p53 is limited in its anti-tumor activities by mutations in the protein itself. Currently, there are greater than 10 million people with tumors that contain inactivated p53, while a similar number have tumors in which the p53 pathway is partially abrogated by inactivation of other signaling components. This has left cancer researchers with the grand challenge of searching for therapies that could restore the protein’s protective function, which Kevetrin appears to be doing the majority of the time.
The clinical trial titled, “A Phase 1, Open-Label, Dose-Escalation, Safety, Pharmacokinetic and Pharmacodynamic Study of Kevetrin (Thioureidobutyronitrile) Administered Intravenously, in Patients With Advanced Solid Tumors,” is available at:http://clinicaltrials.gov/ct2/show/NCT01664000?term=cellceutix&rank=1
About Ovarian Cancer:
Ovarian cancer is the most lethal of the gynecologic cancers, representing the ninth most common cancer among women and the fifth leading cause of cancer related death among women. It most commonly occurs in postmenopausal women. Ninety per cent of ovarian cancers are derived from the ovarian surface epithelium and these neoplasm are classified into serous, mucinous, endometrioid, clear-cell and transitional-cell types. The molecular pathology of ovarian carcinomas is heterogeneous and involves various putative precursor lesions and multiple pathways of development. The most common subtype, high-grade serous carcinoma, is characterized by p53 mutations, and BRCA1 and/or BRCA2 dysfunction.
About Cellceutix:
Headquartered in Beverly, Massachusetts, Cellceutix is a publicly traded company under the symbol “CTIX”. Cellceutix is a clinical stage biopharmaceutical company developing innovative therapies in oncology, dermatology and antimicrobial applications. Cellceutix believes it has a world-class portfolio of compounds and is now engaged in advancing its compounds and seeking strategic partnerships. Cellceutix’s anti-cancer drug Kevetrin is currently in a Phase 1 clinical trial at Harvard Cancer Centers’ Dana Farber Cancer Institute and Beth Israel Deaconess Medical Center. In the laboratory Kevetrin has shown to induce activation of p53, often referred to as the “Guardian Angel Gene” due to its crucial role in controlling cell mutations. Cellceutix will soon begin a Phase 2 clinical trial with its novel compound Brilacidin-OM for the prevention of Oral Mucositis in patients with head and neck cancer. Brilacidin-OM, a defensin mimetic compound, has shown in an animal model to reduce the occurrence of severe ulcerative oral mucositis by more than 94% compared to placebo. Cellceutix’s anti-psoriasis drug Prurisol has recently completed a Phase 1 clinical trial and is being readied for a Phase 2 trial. Prurisol is a small molecule that acts through immune modulation and PRINS reduction. Cellceutix’s lead antibiotic, Brilacidin, has completed a Phase 2b trial for Acute Bacterial Skin and Skin Structure Infections, or ABSSSI. Top-line data have shown a single dose of Brilacidin to deliver comparable clinical outcomes to the FDA-approved seven-day dosing regimen of daptomycin. Brilacidin has the potential to be a single-dose therapy for certain multi-drug resistant bacteria (Superbugs). Cellceutix has formed research collaborations with world-renowned research institutions in the United States and Europe, including MD Anderson Cancer Center, Beth Israel Deaconess Medical Center, and the University of Bologna. More information is available on the Cellceutix web site at http://www.cellceutix.com.
Sign-up for Cellceutix email alerts is available at
http://cellceutix.com/email-alerts/#sthash.VNkn7FY9.dpbs
Forward-Looking Statements
This press release contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 that involve risks, uncertainties and assumptions that could cause Cellceutix’s actual results and experience to differ materially from anticipated results and expectations expressed in these forward looking statements. Cellceutix has in some cases identified forward-looking statements by using words such as “anticipates,” “believes,” “hopes,” “estimates,” “looks,” “expects,” “plans,” “intends,” “goal,” “potential,” “may,” “suggest,” and similar expressions. Among other factors that could cause actual results to differ materially from those expressed in forward-looking statements are Cellceutix’s need for, and the availability of, substantial capital in the future to fund its operations and research and development; including the amount and timing of the sale of shares of common stock to Aspire Capital; the fact that Cellceutix’s compounds may not successfully complete pre-clinical or clinical testing, or be granted regulatory approval to be sold and marketed in the United States or elsewhere. A more complete description of these risk factors is included in Cellceutix’s filings with the Securities and Exchange Commission. You should not place undue reliance on any forward-looking statements. Cellceutix undertakes no obligation to release publicly the results of any revisions to any such forward-looking statements that may be made to reflect events or circumstances after the date of this press release or to reflect the occurrence of unanticipated events, except as required by applicable law or regulation.
Copyright © 2015 Cellceutix Corporation, All rights reserved.
You requested to receive email alerts about Cellceutix at our website http://www.cellceutix.com
Our mailing address is:
Cellceutix Corporation
100 Cumming Center, Suite 151-B
Beverly, MA 01915
Add us to your address book
Email Marketing Powered by MailChimp
unsubscribe from this list | update subscription preferences
Your sentiment is what every cancer researcher hopes and prays! Let’s wait to see some clinical data first.
It impressed me, very much. There is great promise with all of these new molecules – we will have to wait for the data from the clinical trials to pass judgment. I am especially interested in seeing whether patients with mutated p53 benefit. It would make sense that they should because of the multiple ways Kevetrin acts, that is, not just on p53, itself. We will keep an eye on the clinical data, for sure.
Excellent write up on the MOA of Kevetrin. In your opinion what are the odds of this drug curing a majority of cancers based on its p53 expression capabilities? So little news on such a potential game changer for the field of cancer, thoughts?
Many prayers have been answered and millions will be given a second chance at life! This is a giant leap forward in oncology.