Novartis entered into a $90 MM deal with Oxford Biomedica for use of its lentiviral vectors with its CAR (chimeric antigen receptor) modified T-cell product, CTL019, being developed with researchers at UPENN.
CTL019 is a product based on patients autologous T-cells engineered to express receptors (antibody constructs) that recognize CD19 on the surface of B-cells. In clinical studies in highly refractor patients with acute lymphoblastic leukemia who have failed multiple prior chemotherapeutic regimens and bone marrow transplants, 90% of patients have been induced into complete remissions. These results, reported October 16, 20104 in the New England Journal of Medicine, are truly remarkable.
A total of 30 children and adults received CTL019. Complete remission was achieved in 27 patients (90%), including 2 patients with blinatumomab-refractory disease and 15 who had undergone stem-cell transplantation. CTL019 cells proliferated in vivo and were detectable in the blood, bone marrow, and cerebrospinal fluid of patients who had a response. Sustained remission was achieved with a 6-month event-free survival rate of 67% (95% confidence interval [CI], 51 to 88) and an overall survival rate of 78% (95% CI, 65 to 95). At 6 months, the probability that a patient would have persistence of CTL019 was 68% (95% CI, 50 to 92) and the probability that a patient would have relapse-free B-cell aplasia was 73% (95% CI, 57 to 94). All the patients had the cytokine-release syndrome. Severe cytokine-release syndrome, which developed in 27% of the patients, was associated with a higher disease burden before infusion and was effectively treated with the anti–interleukin-6 receptor antibody tocilizumab.
Why lentiviral vectors. Simple – in order for the therapy to broadly applicable, repeatable and efficient methods for transducing the T-cells are required. And, lentiviral vectors, because they are retroviruses, like HIV, are very efficient at transfecting T-cells. For example, look at the diagram below for gene therapy of combined immunodeficiency syndrome in which the lentiviral vector is being used to express cytokine receptors in T-cells.
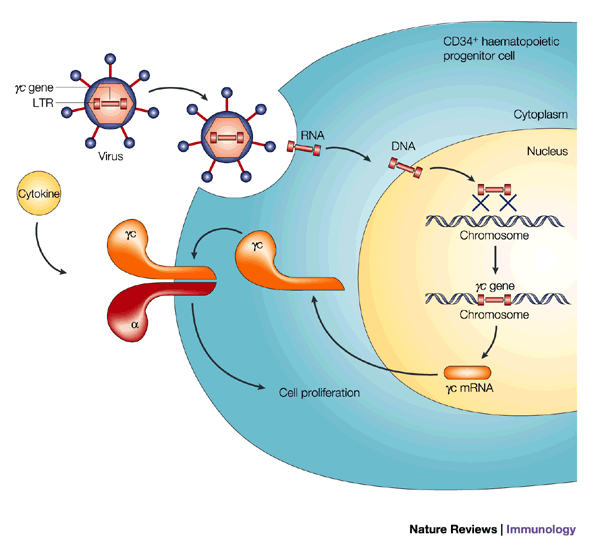
CD34+ cells are incubated ex vivo with supernatant that contains retrovirus encoding the common cytokine-receptor gamma-chain (gammac) gene. Binding of the virus to a cell is followed by viral entry. Viral RNA is retrotranscribed into DNA, which, as a preintegration complex, can recombine with the cell’s genome. The gammac gene can be transcribed, being under the control of the viral long terminal repeat (LTR), which leads to protein synthesis, membrane expression and function. mRNA, messenger RNA. – http://www.nature.com/nri/journal/v2/n8/fig_tab/nri859_F3.html
This construct is easily adapted for the delivery of the chimeric antigen receptor consists of an intracellular T-cell receptor CD3-zeta chain signaling domain that induces T-cell activation, a costimulatory 4-1BB domain that enhances T-cell mediated responses and anti-CD19 antibody fragments that bind to CD19.
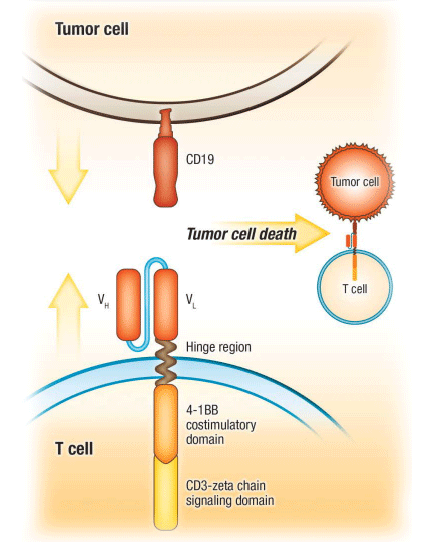
CD19 is widely expressed on B cells, starting from the earliest precursor cells through all stages of maturation. CTL019 therapy involves adoptive transfer of autologous T cells that have been modified to express chimeric antigen receptors designed to recognize and kill CD19+ cancer cells. The chimeric antigen receptor consists of an intracellular T-cell receptor CD3-zeta chain signaling domain that induces T-cell activation, a costimulatory 4-1BB domain that enhances T-cell mediated responses and anti-CD19 antibody fragments that bind to CD19. T cells harvested from a patient are transduced with a lentiviral vector encoding the anti-CD19 chimeric antigen receptor. The resulting CTL019 cells are expanded ex vivo prior to infusion into the patient, who has undergone lymphocyte-depleting therapy. The CTL019 cells destroy tumor cells expressing CD19 and remain persistent in the body to guard against residual or recurring disease. Ongoing studies are investigating the activity and safety of CTL019 therapy in patients with resistant or refractory CD19+ hematologic malignancies. http://hkgalden.com/view/75584

