Approximately 7% of patients with non-small cell lung cancer (NSCLC) possess a transgene that results from an inversion of chromosome 2 that juxtaposes the 5’ end of the echinoderm microtubule-associated protein-like 4 (EML4) gene with the 3′ end of the anaplastic lymphoma kinase (ALK) gene, resulting in the novel fusion oncogene EML4-ALK .

Figure 1. EML4-ALK oncogene: a) wild type EML4 and ALK. b) fused EML4-ALK. http://atlasgeneticsoncology.org/Genes/EML4ID44353ch2p21.html
Anaplastic lymphoma kinase is a 1620 amino acid transmembrane protein that is part of the insulin family of receptor tyrosine kinases:
It contains a short transmembrane domain and an intracellular domain with a juxtamembranous segment with a binding site for insulin receptor substrate-1, a kinase domain with three tyrosine-containing motif (YxxxYY: tyrosines 1278, 1282, and 1283 within the activation loop) and a C-terminus with binding sites for Src homology-2 and phospholipase C-γ. Activation of ALK by binding of ligand leads to dimerization, autophosphorylation, and signaling through the RAS-ERK, JAK3-STAT3, and PI3-K/Akt pathways, which lead to effects on cell proliferation, survival, migration, and alterations in cytoskeletal rearrangement.
In Drosophila, ALK is important for the development of visceral musculature, and for neuronal circuitry in the visual system. In mammals, ALK is present in the nervous system during embryogenesis with reduced levels after birth. ALK knockouts have no gross abnormalities – subtle neurocognitive changes consistent with hippocampal function.
The EML4-ALK translocation results in dysregulated aberrant firing of the ALK tyrosine kinase, which drives the cancer.
Targeted therapy
Xalkori (crizotinib) is an oral tyrosine kinase inhibitor that was approved by FDA in 2011 for the treatment of patients with ALK-positive non-small cell lung cancer (ALK+NCSLC) with metastatic disease. It is an inhibitor of receptor tyrosine kinases including ALK, Hepatocyte Growth Factor Receptor (HGFR, c-Met), ROS1 (c-ros), and Recepteur d’Origine Nantais (RON).
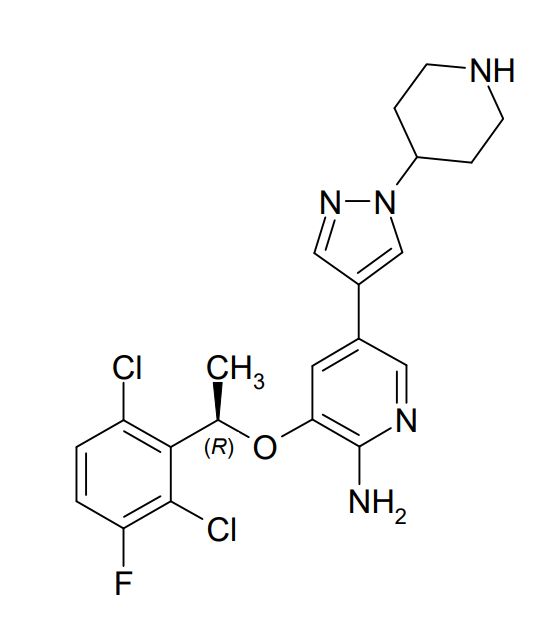
Figure 2. Chemical structure of crizotinib (Xalkori). https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/202570s016lbl.pdf
In a study in patients with newly diagnosed ALK+ NSCLC, crizotinib was shown to be superior to chemotherapy (pemetrexed 500 mg/m2 with cisplatin 75 mg/m2 or carboplatin).
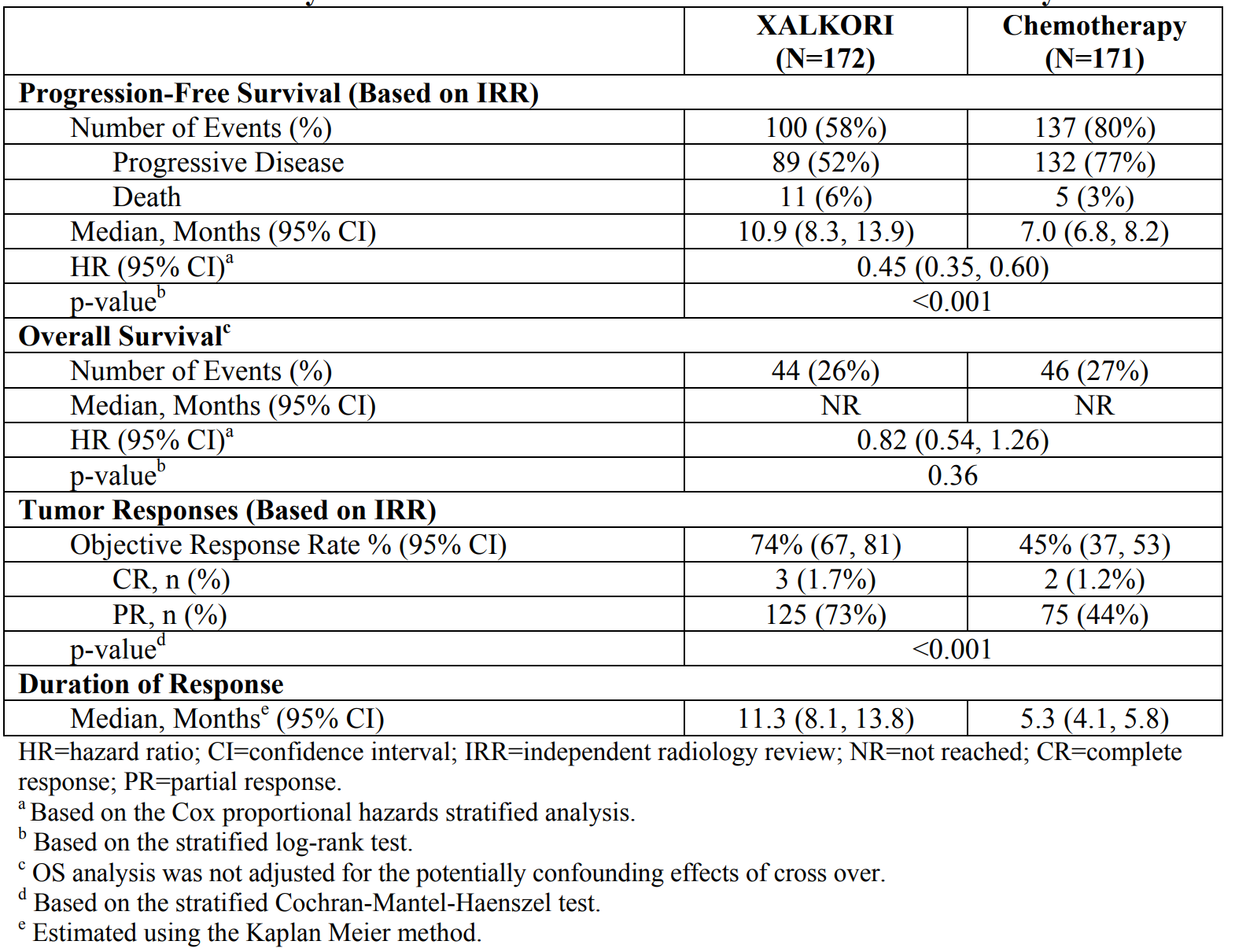
Table 1. Crizotinib in front-line ALK+ NSCLC versus chemotherapy. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/202570s016lbl.pdf
Although the majority of patients initially do well on crizotinib, responses last for only about a year. Alecenza (alectinib) is a second generation kinase inhibitor indicated for the treatment of patients with anaplastic lymphoma kinase (ALK)-positive, metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib. It inhibits ALK and RET, a cell surface receptor tyrosine kinase that binds glial cell line-derived neurotrophic factors – GDNFs.
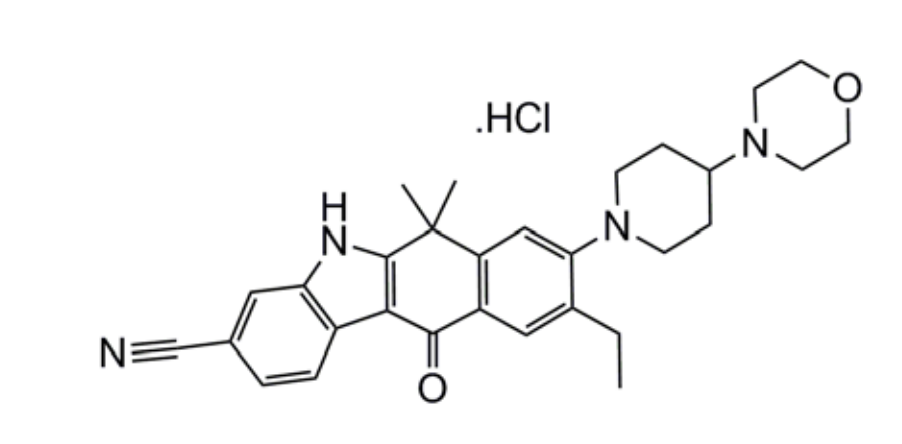
Figure 3. Chemical structure of alectinib. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/208434s000lbl.pdf
In two non-randomized studies, alectinib demonstrated a response rates of 46% and 48%. In a combined analysis of patients with CNS metastases, a 61% objective response rate (18% complete response and 43% partial response) was seen. The drug was approved by the FDA in 2015.
Alectinib versus crizotinib, front-line
An open-label study (ALEX) randomized 303 treatment-naive patients with ALK-positive NSCLC in a 1:1 ratio to alectinib or crizotinib. PFS (progression-free survival) was the primary endpoint of the study, which was conducted at 161 locations across 31 countries:
- The primary endpoint, median PFS as assessed by independent review, was significantly improved with alectinib. Median PFS not reached in the alectinib arm, with a lower confidence interval of 20.3 months, compared with a median PFS of 10.2 months in the crizotinib arm (HR, 0.34; P <.0001).
- In the subgroup of patients with brain metastases at baseline, the hazard ratio for the primary endpoint with alectinib versus crizotinib was 0.08 (95% CI, 0.01-0.61).
- Objective response rates as determined by investigators were 85.4% in the alectinib group and 70.2% in the crizotinib group. As assessed by independent review, ORR was 91.6% in the alectinib arm and 78.9% in the crizotinib arm.
- Grade 3/4 AEs occurred with greater frequency in the crizotinib arm compared with the alectinib arm (51.9% vs 26.2%, respectively). Discontinuation of study drug due to AEs occurred more frequently in the crizotinib arm compared with the alectinib arm (20.2% vs 8.7%, respectively), as did dose interruption due to AEs (74.0% vs 29.1%, respectively).
Alectinib is now the front-line drug of choice in newly diagnosed patients. Since crizotinib is also a MET inhibitor, it will have a role in patients who progress following treatment with alectinib, if MET is mediating resistance. Another second generation ALK inhibitor, Alunbrig (brigatinib), for patients with metastatic ALK-positive NSCLC who have progressed on or are intolerant to crizotinib was approved earlier this year.
