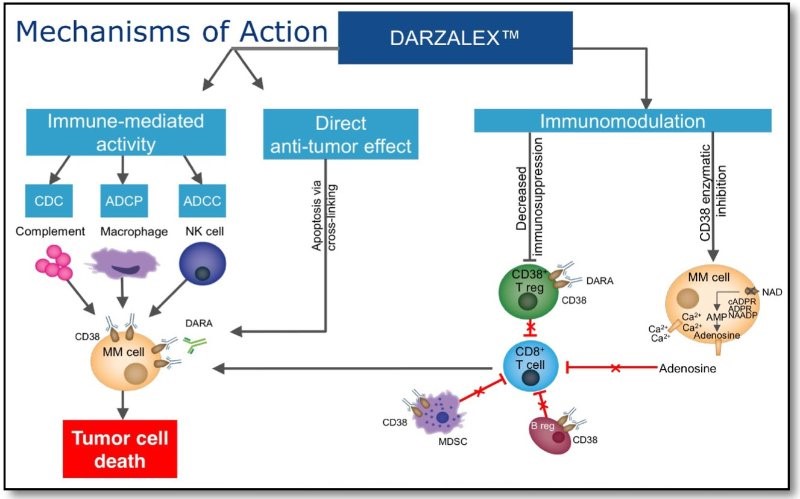
We have previously reviewed anti-CD38 monoclonal antibody Darzalex (daratumumab) in multiple myeloma. Daratumumab targets CD38-expressing myeloma cells through a variety of immune-mediated mechanisms (complement-dependent cytotoxicity, antibody-dependent cell-mediated cytotoxicity, and antibody-dependent cellular phagocytosis) and direct apoptosis with cross-linking of receptors.
It received accelerated approval by the FDA in November 2015 for the treatment of patients with multiple myeloma who have received at least three prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory agent or who are double-refractory to a PI and an immunomodulatory agent. It was shown in the SIRIUS trial to have good single-agent activity against heavily pretreated relapsed/refractory multiple myeloma.
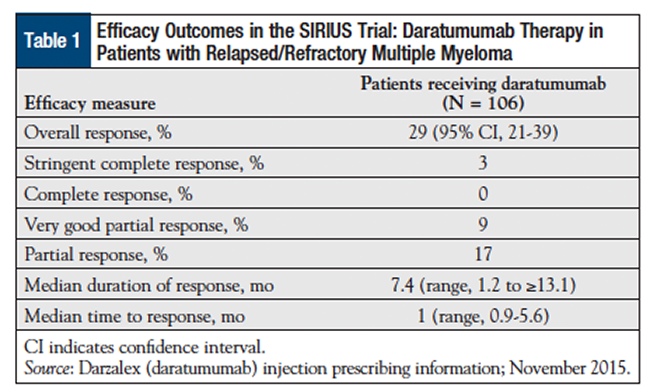
The overall response rate was 29%, which included 3% stringent complete responses, 9% very good partial responses, and 17% partial responses. The median time to disease progression was 3.7 months, and the median overall survival has not been reached. http://oncpracticemanagement.com/issue-archive/2016/april-2016-vol-6-no-4/darzalex-daratumumab-first-anti-cd38-monoclonal-antibody-approved-patients-relapsed-multiple-myeloma/
Clinical Results
Additionally, data from the GEN503 study, presented at the 2015 American Society of Hematology annual meeting, showed that daratumumab added to standard therapy with lenalidomide (Revlimid) and dexamethasone (Rd combination) produced high response rates in patients for whom at least one prior line of therapy, including a proteasome inhibitor and/or an immunomodulator, had failed.
The lead investigator, Torben Plesner, MD, professor of hematology at the University of Southern Denmark in Velje, reported that the responses were durable and occurred rapidly, with a median time to first response of one month and median time to best response of 5.1 months. As of the time of the report in December 2015, the median duration of response had not been reached, and at one-year follow-up, 91% of those patients who had achieved at least a partial response did not have disease progression.
Also in the relapsed/refractory setting, daratumumab plus standard of care (lenalidomide and dexamethasone or bortezomib and dexamethasone [Vd]) versus Rd or Vd alone in patients with relapsed or refractory multiple myeloma is being explored in the Castor and Pollux trials.
The antibody is also being studied for use in first-line therapy for patients with newly diagnosed active multiple myeloma with measurable disease who are ineligible for high-dose induction chemotherapy and autologous stem cell transplantation
New mechanism of action identified
Now, a team of investigators is reporting that in addition to direct targeting of CD38-positive cells, daratumumab may exert indirect effects on multiple myeloma by allowing an increase in T-helper cells, cytotoxic T-lymphocytes, T-cell function response, and T-cell receptor clonality. In a study published in Blood, investigators examined peripheral blood and bone marrow samples collected before and during therapy and following relapse in 148 patients with relapsed/refractory multiple myeloma who had been treated in one of two studies of daratumumab monotherapy.
The researchers looked for daratumumab sensitivity and immunosuppressive activity in two cell populations known to express CD38: regulatory B cells and myeloid-derived suppressor cells (MDSCs). In doing so, Krejcik and colleagues identified a previously undocumented subpopulation of regulatory T cells (Tregs) that express CD38 at high levels, which showed a “significant and almost immediate decline,” they said, following the first dose of daratumumab. The cells remained depleted throughout the course of treatment with daratumumab, suggesting that the drug can dampen the immunosuppressive effects of Tregs.
The team also found that the antibody induces the expansion of helper and cytotoxic T cells, “skews the T-cell repertoire,” and increases the clonality of T-cell receptors, implying that the drug fosters improvement in the patient’s adaptive immune response.
The authors noted that the reductions in MDSCs, Tregs, and B-regulatory cells they saw occurred in concert with an expansion of CD4+ T-helper cells and CD8+ cytotoxic T-cells, and that T-cell clonality and functional anti-viral responses also increased with the drug.
CD38 Expression in hematopoietic cells
In 1988, the Irving Weissman lab at Stanford made the first hierarchical hematopoietic differentiation scheme of development in mice. It has been adapted for humans and currently is depicted as:
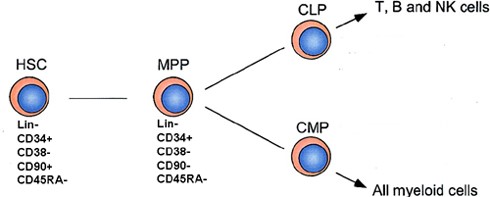
HSC (hematopoietic stem cell), MPP (multipotent progenitors), CLP (Common Lymphoid Progenitor), CMP (Common Myeloid Progenitor). http://hematopoiesis.info/2008/01/21/hierarchy-of-human-hematopoesis-scheme-is-updated/
Thus, CD38 is expressed in T-cells, B-Cells, and myeloid cells (dendritic cells, monocytes, macrophages, and granulocytes).
CD38 Ecto-enzyme generating adenosine in the tumor microenvironment
In addition to being a receptor, CD38 is also an enzyme – it is a component of a pathway leading to the production of adenosine in the tumor microenvironment, thus inducing local anergy.

The adenosine pathway participates in the creation of an immune-tolerant tumour microenvironment by regulating the functions of immune and inflammatory cells, such as macrophages, dendritic cells, myeloid-derived suppressor cells, T cells and natural killer (NK) cells. The adenosine pathway also regulates cancer growth and dissemination by interfering with cancer cell proliferation, apoptosis and angiogenesis via adenosine receptors that are expressed on cancer cells and endothelial cells, respectively. Solid tumours express high levels of CD39 and CD73, as well as low levels of nucleoside transporters (NTs), ecto-adenosine deaminase and its cofactor CD26, which lead to an increase in adenosine signalling in the cancer environment. ADA, adenosine deaminase; JNK, JUN amino-terminal kinase; TH1, T helper 1; TReg, regulatory T; VEGF, vascular endothelial growth factor. http://www.nature.com/nrc/journal/v13/n12/fig_tab/nrc3613_F2.html
Consequently, not only might CD38 be a prime target for MAb-mediated therapy, but its functional block (leading to decreased levels of adenosine in the tumor microenvironment) may contribute to general improvement in cancer immunotherapy and outcomes.
Indeed, that was seen with datatumumab treatment:
Krejcik and colleagues identified a previously undocumented subpopulation of regulatory T cells (Tregs) that express CD38 at high levels, which showed a “significant and almost immediate decline,” they said, following the first dose of daratumumab. The cells remained depleted throughout the course of treatment with daratumumab, suggesting that the drug can dampen the immune-suppressive effects of Tregs.
The team also found that the antibody induces the expansion of helper and cytotoxic T cells, “skews the T-cell repertoire,” and increases the clonality of T-cell receptors, implying that the drug fosters improvement in the patient’s adaptive immune response.
The authors noted that the reductions in MDSCs, Tregs, and B-regulatory cells they saw occurred in concert with an expansion of CD4+ T-helper cells and CD8+ cytotoxic T-cells, and that T-cell clonality and functional anti-viral responses also increased with the drug.
“These observations indicate that T cells continued to function properly, despite low CD38 expression, and suggest that increased T-cell response may be due to depletion of regulatory cells. Further, these changes in T-cell expansion, activity, and clonality were more pronounced in patients who responded to daratumumab compared with those who did not,” they wrote.
Additionally, many of the changes observed were reversed in patients who experienced disease relapse while on daratumumab.
“This suggests an additional, previously uncharacterized mechanism of action of daratumumab through immunomodulation that may contribute to clinical responses and its efficacy in such a heavily treated patient population,” the researchers wrote.
Conclusion
So, multiple mechanisms of action are responsible for the excellent clinical results seen with daratumumab: (1) effects on myeloma cells including antibody mediated CDC (complement dependent cytotoxicity), ADCP (antibody dependent cellular phagocytosis), ADCC (antibody dependent cellular cytotoxicity), apoptosis mediated by receptor cross-linking; (2) killing of Treg and MDSC (myeloid derived suppressor cells) that express CD38 by the same mechanisms; and (3) immunomodulation mediated by decreased adenosine in the tumor microenvironment.