Antibody Dependent Cell-Mediated Cytotoxicity (ADCC) is a process by which the Fab (variable region) of antibodies, produced by B-cells, first bind to antigens on target cells (cancer), and their Fc (constant regions) then bind white cells (macrophages, granulocytes, and NK – natural killer cells), which destroy the target cells. The antibodies opsonize the target and then attract white cells to destroy the target.Novartis just signed a research and development agreement with a company called ProBioGen that has GlymaxX Antibody Glyco-Engineering Technology to enhance the ADCC potential of monoclonal antibodies. The technology involves removing the sugar fucose from the CH2 domain of the Fc region. This allows the CH2 domain to bind with the CD16 receptor on natural killer cells more avidly, resulting in a more potent ADCC response. (See diagram from Discovery Medicine.)
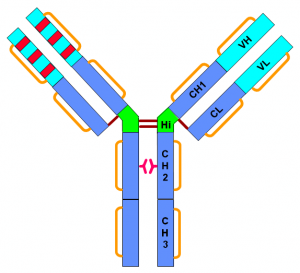
Figure 1. Structure of humanized IgG1. All current approved therapeutic antibodies are IgGs or their derivatives. Human G immunoglobulins are tetrameric glycoproteins (150 kDa) composed of two heavy chains (HC, 50 kDa) and two light chains (LC, 25 kDa). They are divided in four subclasses or isotypes defined by different heavy chains (γ1, γ2, γ3, and γ4). They share greater than 90% sequence homology in their constant domains but differ significantly in the hinge region. Disulfide bridges (sixteen for IgG1 and IgG4; eighteen for IgG2) and non-covalent interactions maintain their 3-dimensional structure. The heavy and light chains are linked by one disulfide bond and the heavy chains by two (for IgG1 and IgG4) or three (for IgG2) disulfide bonds, all located in the small hinge domain, which also contains a papain cleavage site yielding two Fab and one Fc fragments (50 kDa each). The other twelve or fourteen cysteine bridges are intramolecular and delimit six different globular domains: one variable (VL) and one constant (CL) for the light chain; one variable (VH) and three constant (CH1, CH2, and CH3) for the heavy chain. Each variable domain contains also 3 Complementary Determining Regions (CDRs), a short sequence up to 13 amino acids. The CDRs are the most variable parts of IgGs and contribute to their diversity by making contacts with a specific antigen, allowing IgGs to recognize a vast repertoire of antigens with a high affinity (Beck et al., 2009).
Xenocor is another company developing Fc-optimization technology to enhance ADCC. Their approach involves two amino acid substitutions in the Fc portion of antibodies to increase the avidity of binding to CD16 of natural killer cells.
